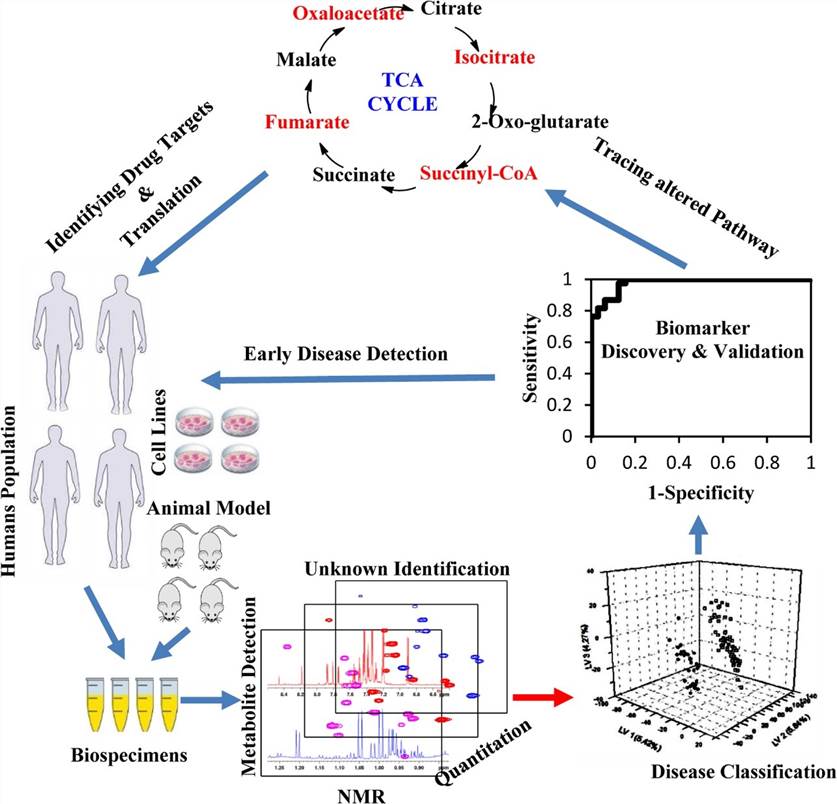
High-Resolution Magic Angle Spinning (HRMAS) NMR spectroscopy is a powerful analytical technique that bridges the gap between solution-state NMR and traditional histopathology. By enabling non-destructive, high-resolution metabolomic analysis of intact tissue specimens, HRMAS allows biochemical characterization while preserving morphological integrity. This capability is especially valuable in cancer research, where quantitative metabolic profiling of biopsy or surgical specimens can complement histopathological assessment, support biomarker discovery, and potentially inform diagnosis, grading, and therapeutic decision-making.
Fundamentals of NMR and HRMAS
Brief Overview of NMR
Nuclear Magnetic Resonance (NMR) spectroscopy is founded on the principle that certain atomic nuclei (e.g., 1H, 13C, 15N) possess intrinsic magnetic moments. In a strong external magnetic field (B0), these nuclear spins align along or against the field and can be perturbed by radiofrequency (RF) pulses. The resulting emitted electromagnetic signal is detected and Fourier-transformed into a spectrum, wherein chemical shifts, coupling patterns, and relaxation times reveal molecular identities, structure, dynamics, and environment.
A primary limitation of conventional NMR is its relatively low sensitivity. Typically, milligram quantities and mM concentrations are required to obtain adequate signal intensity. Moreover, tissue extracts destroy morphological context and may lose labile metabolites.
Magic Angle Spinning (MAS) and HRMAS
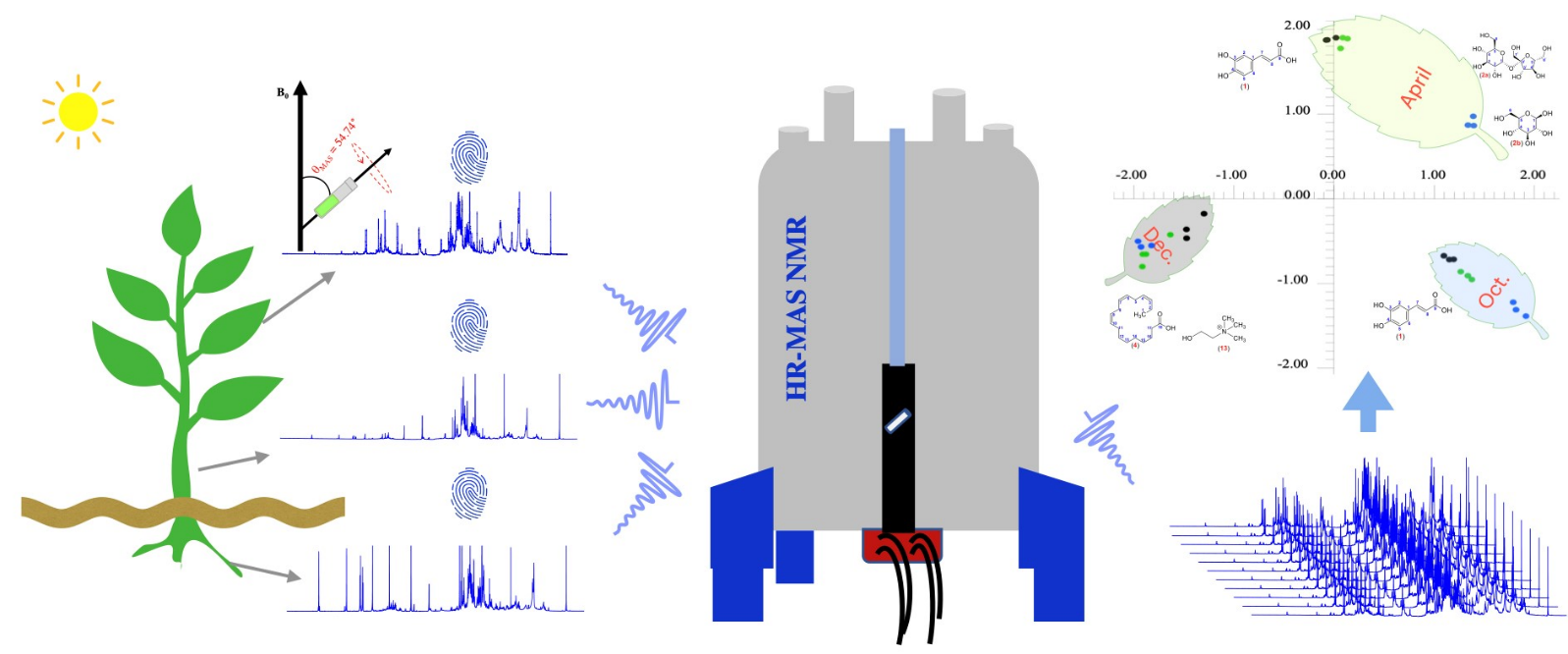
In solids or semi-solid specimens, line broadening arises from anisotropic interactions such as dipolar couplings, chemical shift anisotropy, and quadrupolar couplings, which obscure spectral resolution. Magic Angle Spinning (MAS) mitigates these effects by rotating the sample about an axis set at approximately 54.74° (the "magic angle") relative to B0, thereby averaging orientation-dependent interactions.
High-Resolution Magic Angle Spinning (HRMAS) adapts this concept to intact, hydrated tissue samples—typically biopsy cores or small surgical specimens. By spinning at rates of ~1–5 kHz at low temperature (e.g., 2–4 °C), HRMAS produces spectra with resolution comparable to extracts, while preserving tissue architecture for histopathological correlation.
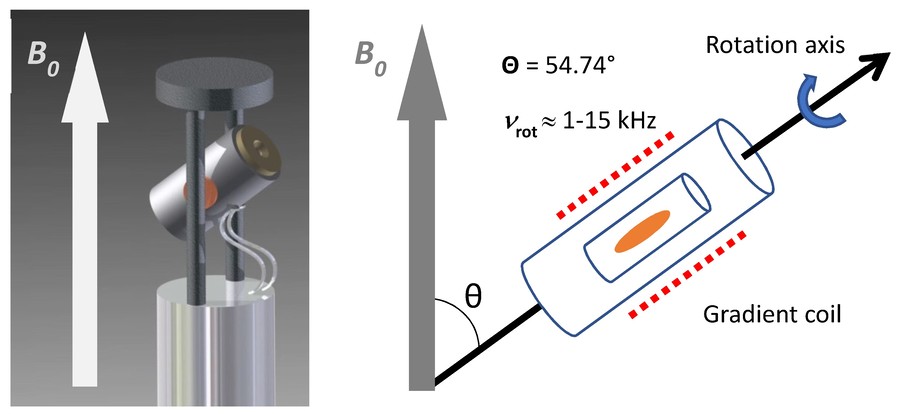 Figure 1. Representation of an HR-MAS NMR probe head with an orientation of the stator at an angle (θ) of 54.7° between axis of rotation and B0. The rotation speed (vrot) of the sample reaches up to 15 kHz. A gradient coil is arranged around the rotor. (Gogiashvili et al., 2019)
Figure 1. Representation of an HR-MAS NMR probe head with an orientation of the stator at an angle (θ) of 54.7° between axis of rotation and B0. The rotation speed (vrot) of the sample reaches up to 15 kHz. A gradient coil is arranged around the rotor. (Gogiashvili et al., 2019)
HRMAS NMR as a Non-Destructive Cancer Tissue Analysis Tool
HRMAS NMR analysis preserves the sample for subsequent histological assessments, enabling direct correlation between tissue morphology and metabolite profiles. This is uniquely powerful in oncology research and diagnostics.
- HRMAS enables metabolic characterization of tumor heterogeneity, cellularity, necrosis, and grade in a manner directly linked to histopathology.
- Tissue cores (often ≤10 mg) are sufficient for standardized protocols, minimizing invasiveness and preserving material for downstream analyses.
As a practical, non-destructive technique, HRMAS is ideal for ex vivo assessment of biopsy specimens, frozen or fresh, without compromising diagnostic or research workflows.
Applications of HRMAS NMR in Cancer Tissue Analysis
HRMAS has been applied across a range of human malignancies, revealing metabolomic biomarkers and discriminative signatures with clinical relevance.
Brain Tumors
In pioneering work, Cheng et al. applied HRMAS 1H spectroscopy to intact human brain tumor specimens (glioblastomas, astrocytomas, meningiomas, and schwannomas). They quantified concentrations and relaxation times for over a dozen metabolites and assessed metabolite-to-creatine ratios. These metabolic parameters correlated significantly with tumor type and grade, and inositol-to-creatine ratios distinguished tumor subtype—all in the same intact tissue cores used for subsequent histology.
Quantitative metabolic profiling via HRMAS allowed detection of biochemical heterogeneity within tumor cores, as confirmed by histopathology. Ratios such as phosphorylcholine to choline correlated with cellular density, while lactate and mobile lipid content tracked necrosis, providing metabolic markers that reflect histological features.
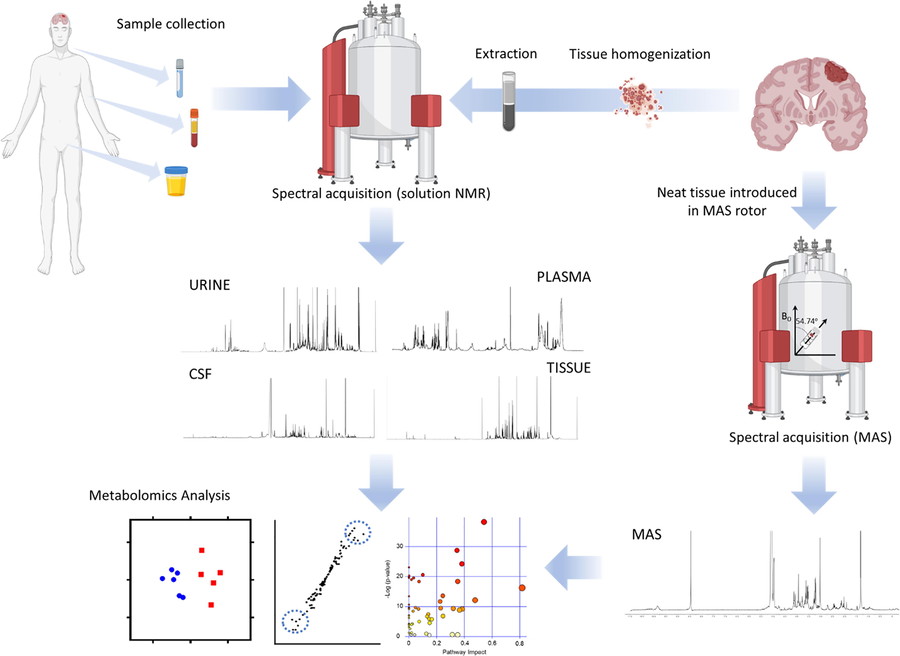 Figure 2. Workflow for metabolomics investigations of brain tumors. (Ruiz-Rodado et al., 2020)
Figure 2. Workflow for metabolomics investigations of brain tumors. (Ruiz-Rodado et al., 2020)
Prostate Cancer
HRMAS has been extensively applied to prostate biopsy specimens. In one study, core needle biopsies obtained from over 200 patients were analyzed via HRMAS 1H NMR, followed by histological evaluation. Specific metabolic signatures—including levels of citrate, glycerol-3-phosphocholine, glycine, carnitine, and phosphocholine—distinguished malignant from benign tissues. Integration with clinical variables (e.g. prostate volume, digital rectal exam findings) yielded predictive models with AUC ~0.87, specificity ~94% and positive predictive value ~80%.
Additionally, metabolic ratios such as total choline/citrate correlated with Gleason score, indicating HRMAS metabolite profiling can support tumor grading insights from minimal tissue cores collected during routine biopsy procedures.
Ex vivo metabolic analysis of biomarkers predictive of prostate cancer recurrence following radical prostatectomy.
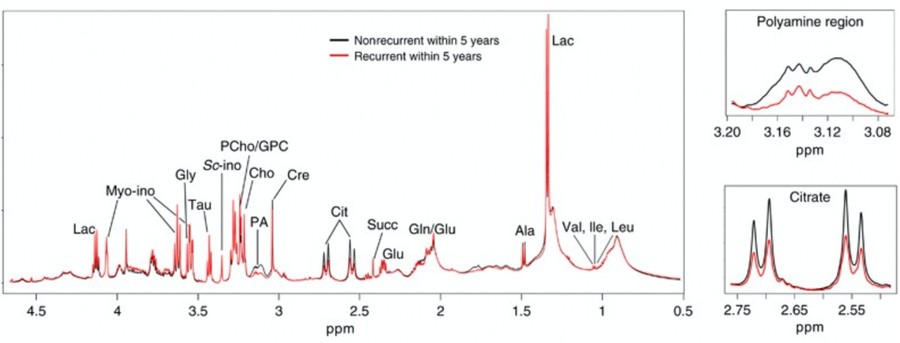 Figure 3. Average high-resolution magic angle spinning (HR MAS) MR spectra of tumors from nonrecurrent (black) and recurrent (red) prostate cancer groups. Magnifications of the polyamine (containing spermine and putrescine) and citrate regions are shown. (Penet et al., 2023)
Figure 3. Average high-resolution magic angle spinning (HR MAS) MR spectra of tumors from nonrecurrent (black) and recurrent (red) prostate cancer groups. Magnifications of the polyamine (containing spermine and putrescine) and citrate regions are shown. (Penet et al., 2023)
Breast Cancer
1H HRMAS spectra on intact breast cancer tissue specimens have displayed rich metabolite assignments: more than 30 metabolites—including choline-containing compounds, taurine, glycine, glutamate, and various lipids—were identified with resolution comparable to extract NMR spectra. Malignant tissues exhibited reduced lipid signals and elevated small-molecule metabolites relative to benign tissues. Multivariate analysis allowed discrimination between malignant and benign samples.
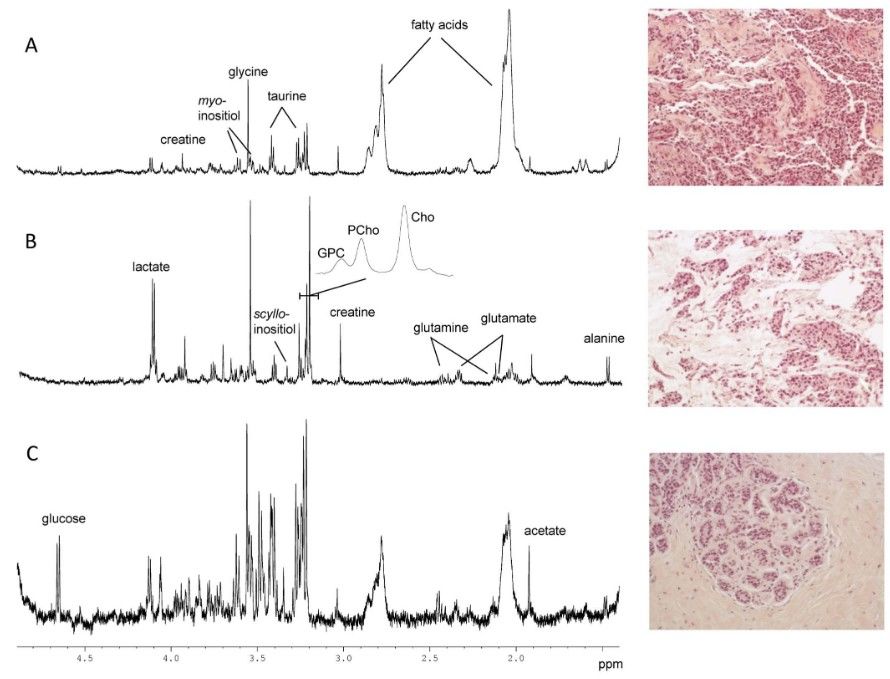 Figure 4. HR MAS spectra and illustration of typical features observed in the corresponding HES images (200X). (A) Invasive ductal carcinoma with an estimated tumor content of 80% in the analyzed biopsy. (B) Invasive mucinous carcinoma with an estimated tumor content of 60% in the analyzed biopsy. (C) Normal breast tissue (adjacent to tumor). No tumor cells were detected, and the HES image shows the typical feature of normal terminal lobular duct units. The poor signal to noise ratio in this spectrum is probably due to the high level of connective tissue (85%). (Bathen et al., 2013)
Figure 4. HR MAS spectra and illustration of typical features observed in the corresponding HES images (200X). (A) Invasive ductal carcinoma with an estimated tumor content of 80% in the analyzed biopsy. (B) Invasive mucinous carcinoma with an estimated tumor content of 60% in the analyzed biopsy. (C) Normal breast tissue (adjacent to tumor). No tumor cells were detected, and the HES image shows the typical feature of normal terminal lobular duct units. The poor signal to noise ratio in this spectrum is probably due to the high level of connective tissue (85%). (Bathen et al., 2013)
Endometrial Cancer
In a comparative HRMAS metabolomics study of endometrial tissues, patients with endometrial cancer demonstrated elevated levels of lactate, glucose, phosphocholine, glycerophosphocholine, amino acids (leucine, glycine, valine, methionine, etc.), taurine, and alanine relative to controls. PCA and PLS-DA analyses clearly separated cancerous from non-cancerous tissues, demonstrating HRMAS's capacity to discern disease-specific metabolic phenotypes from intact samples.
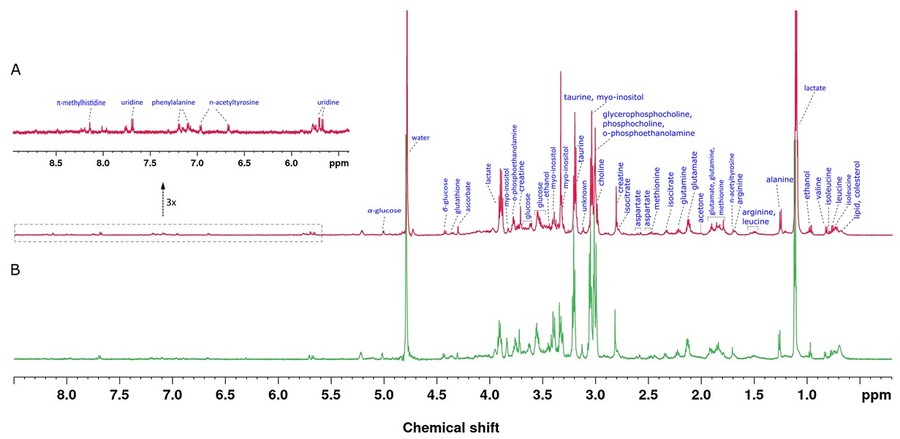 Figure 5. HR-MAS NMR spectra of tissue samples obtained from the study subjects acquired by a 600 MHz NMR spectrometer using a CPMG (Carr-Purcell-Meiboom-Gill) pulse sequence at a spinning speed of 3.5 kHz. 1H NMR spectrum of endometrium cancer tissue (A) and 1H NMR spectrum of healthy control tissue (B). (Arda et al., 2022)
Figure 5. HR-MAS NMR spectra of tissue samples obtained from the study subjects acquired by a 600 MHz NMR spectrometer using a CPMG (Carr-Purcell-Meiboom-Gill) pulse sequence at a spinning speed of 3.5 kHz. 1H NMR spectrum of endometrium cancer tissue (A) and 1H NMR spectrum of healthy control tissue (B). (Arda et al., 2022)
Lung and Other Cancers
HRMAS 1H NMR of lung tumor specimens and adjacent normal parenchyma effectively discriminated tumor from non-tumor tissues with ~95% sensitivity and 100% specificity. Metabolites elevated in tumors included lactate, glycerophosphocholine, phosphocholine, taurine, reduced glutathione, and UDP, whereas glucose, amino acids, acetate, and inositols were reduced. Differentiation among tumor histotypes (adenocarcinoma, carcinoid, squamous carcinoma) was also achievable via metabolic profiles.
HRMAS has similarly been applied in studies of pancreatic cancer (xenograft models), cervical cancer, kidney cancer, lymph nodes, and liposarcoma, demonstrating broad adaptability in oncology metabolomics.
Methodology: HRMAS Workflow for Cancer Tissue Analysis
The HRMAS experimental workflow typically includes:
Tissue Collection and Preparation
- Small tissue specimens (1–10 mg) are excised—either from surgical resection or biopsy.
- Samples are cooled (≈4 °C) and stored briefly prior to analysis.
- Minimal sample preparation: tissues are placed in HRMAS rotors with D2O or buffer for locking; no extraction required. This preserves metabolites and tissue structure.
Instrumental Setup
- HRMAS experiments are conducted on high-field NMR spectrometers (400–800 MHz for 1H).
- MAS spinning rates of ~2 kHz reduce line broadening; temperature is controlled at ~2–5 °C to maintain metabolic state.
- Typical experiments include 1D PRESS, 2D J-resolved, and TOCSY or HSQC as appropriate.
Spectral Acquisition and Processing
- Spectra are recorded across multiple samples.
- Pre-processing includes baseline correction, phasing, and normalization.
- Spectral bins or integrals of metabolite peaks are quantified.
Statistical Analysis
- Multivariate statistical methods such as PCA, PLS-DA, or OPLS-DA are employed to identify discriminative metabolic features.
- Metabolite ratios (e.g. choline/creatine, lactate/citrate) and absolute concentrations are correlated with pathology (e.g., cancer vs. benign, grade, subtypes).
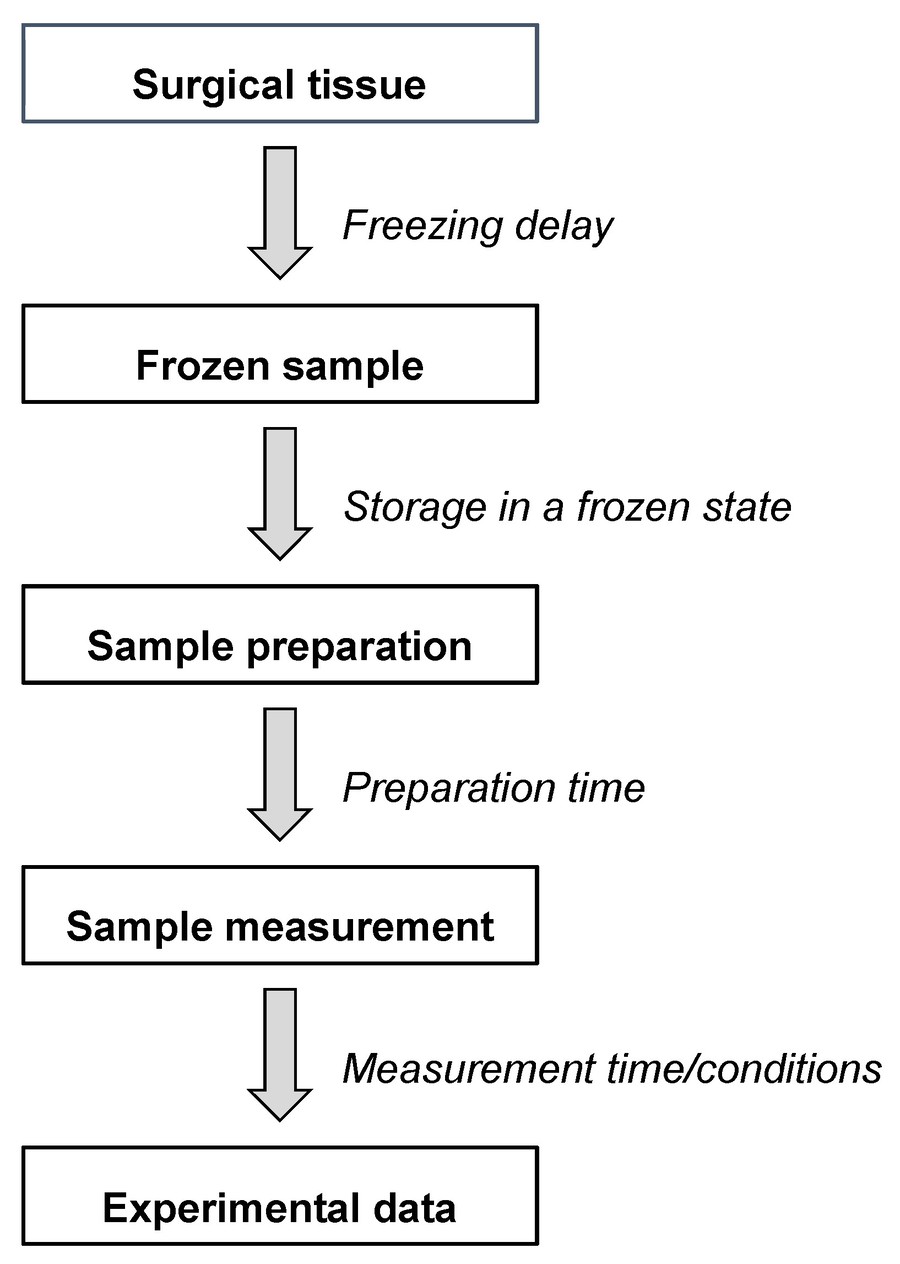 Figure 6. Different steps and time windows between the tissue sampling and the final HR-MAS NMR data. (Gogiashvili et al., 2019)
Figure 6. Different steps and time windows between the tissue sampling and the final HR-MAS NMR data. (Gogiashvili et al., 2019)
Histopathological Correlation
- After HRMAS analysis, the same tissue samples undergo histopathological staining (e.g., H&E).
- Histology and metabolic data are correlated on a per-sample basis to validate biochemical findings against tissue phenotype.
This integrated workflow combines molecular and morphological data from the same specimen—maximizing insight while minimizing tissue use.
Key Metabolite Markers in Cancer by HRMAS
HRMAS studies across cancer types consistently identify a core set of metabolites associated with malignancy:
- Choline-Containing Compounds (phosphocholine, glycerophosphocholine, total choline): Elevated in malignant tissues due to membrane turnover and proliferation.
- Lactate: Elevated glycolytic activity typical of tumors.
- Lipids: Alterations in mobile lipid signals reflect necrosis and cell death.
- Citrate: Often decreased in prostate cancer.
- Amino Acids (e.g., glycine, taurine, glutamate, valine, leucine): Reflect altered metabolism.
- Glucose, Inositols: Reduced in many tumor tissues relative to benign counterparts.
Ratios between these metabolites and creatine or total choline yield clinically relevant predictive metrics—e.g., choline/citrate ratio correlates with prostate tumor presence and grade.
Select Service
Case Studies
Case 1: Metabolomic prediction of oral SCC malignancy using ¹H HRMAS NMR
Oral squamous cell carcinoma (OSCC) is a major global health concern, often linked to tobacco and alcohol use. This study employed 1H High-Resolution Magic Angle Spinning (HRMAS) NMR spectroscopy to develop precise metabolic profiles and statistical models for malignancy prediction in OSCC tissue samples—including tumor, margin, bed, and facial muscle. Spectral data from 180 tissues of 43 patients were analyzed, and three predictive models were generated based on metabolites and lipids. These models were then tested blindly on 64 tissues from 12 OSCC patients with neck invasion, achieving over 90% accuracy. The findings highlight HRMAS NMR's potential in non-invasive malignancy prediction, with implications for real-time surgical guidance and machine learning integration for improved cancer diagnostics.
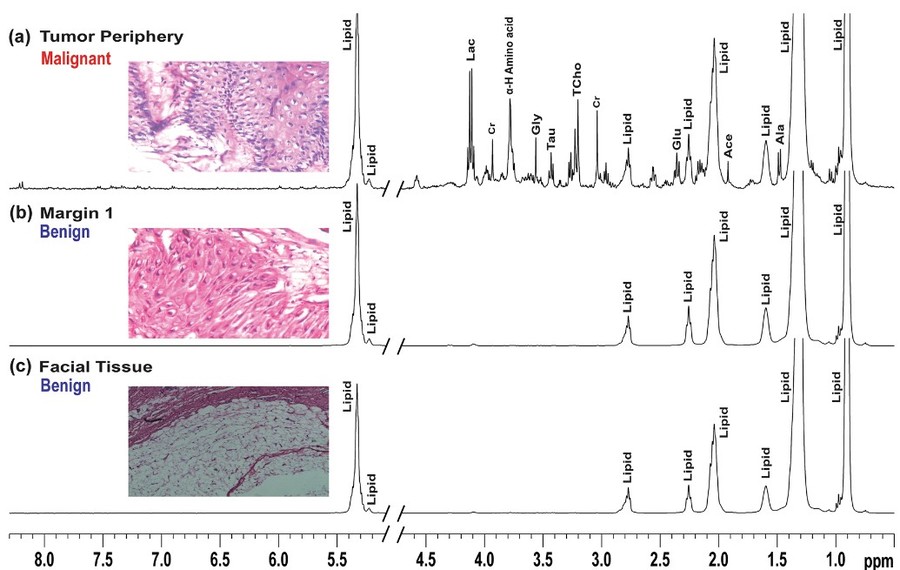 Figure 7. Representative stack plots of HRMAS 1H CPMG NMR spectra recorded on tissues specimens received from patient 1 along with respective histopathological status. The CPMG spectra of tumor and adjacent oral tissues as (top): a tumor periphery (malignant), b lateral margin (benign), c facial tissues (benign). (Paul et al., 2020)
Figure 7. Representative stack plots of HRMAS 1H CPMG NMR spectra recorded on tissues specimens received from patient 1 along with respective histopathological status. The CPMG spectra of tumor and adjacent oral tissues as (top): a tumor periphery (malignant), b lateral margin (benign), c facial tissues (benign). (Paul et al., 2020)
Case 2: Spermine and citrate as metabolic biomarkers for assessing prostate cancer
This study addresses the clinical need to distinguish aggressive from indolent prostate cancer, aiding in treatment decisions and avoiding overtreatment. Researchers analyzed metabolic profiles of 158 prostate tissue samples from 48 patients using high-resolution magic angle spinning (HR-MAS) NMR spectroscopy. Comparing different Gleason scores (GS), they found that high-grade cancers (GS ≥ 7) had significantly lower levels of spermine and citrate, along with higher (choline + creatine + polyamines)/citrate (CCP/C) ratios. These metabolic markers correlated strongly with GS (r = 0.71), enabling classification of cancerous tissue with 86.9% sensitivity and 85.2% specificity. The findings suggest metabolic profiling via HR-MAS NMR or in vivo MRS could serve as a valuable tool in assessing prostate cancer aggressiveness and guiding clinical management.
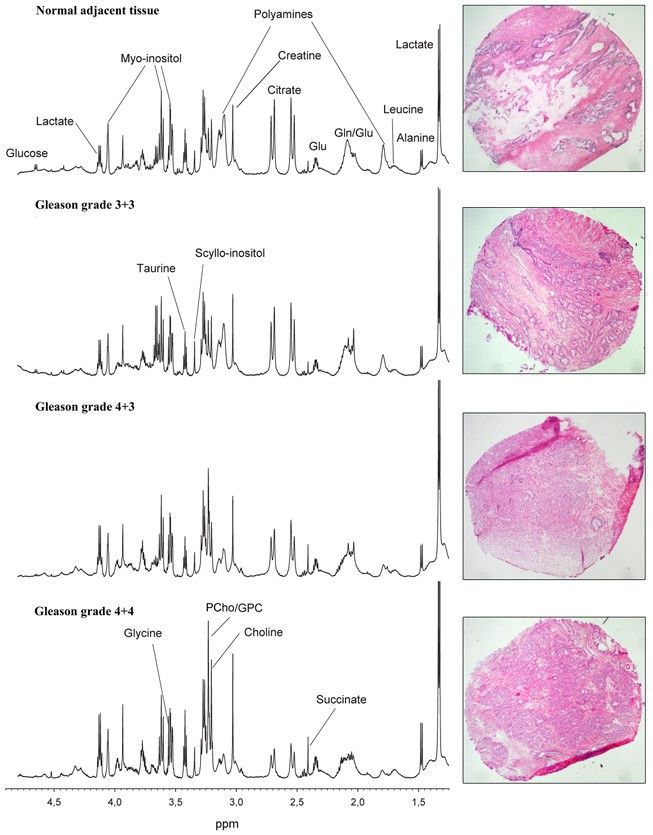 Figure 8. Representative HR-MAS spectra and corresponding HES-stained prostate tissue samples with different Gleason grades. Visual inspection of the spectra shows decreased levels of polyamines (predominately spermine) and citrate, and increased levels of GPC, PCho, and Cho with increasing tumor grade. (Giskeødegård et al., 2013)
Figure 8. Representative HR-MAS spectra and corresponding HES-stained prostate tissue samples with different Gleason grades. Visual inspection of the spectra shows decreased levels of polyamines (predominately spermine) and citrate, and increased levels of GPC, PCho, and Cho with increasing tumor grade. (Giskeødegård et al., 2013)
Advantages of HRMAS-NMR in Cancer Research
- Non-Destructive Nature: HRMAS preserves tissue architecture, enabling simultaneous spectroscopic and histological examination of the identical specimen—a major advantage over extraction-based metabolomics.
- Minimal Sample Requirement: Biopsies or surgical cores of only a few milligrams suffice for high-quality spectral data, enabling multiple analyses from a single specimen.
- High Spectral Resolution with Minimal Processing: HRMAS achieves resolution comparable to extract NMR, allowing detection of both aqueous and lipid-soluble metabolites in a single experiment, without complex extraction protocols.
- Histopathological Correlation: The ability to analyze metabolites and then subject the same sample to histopathology enables direct linkage between metabolic signatures and tumor grade, cellularity, necrosis, or subtype.
- Versatility Across Cancer Types: HRMAS has been successfully applied to prostate, brain, breast, endometrial, lung, cervical, pancreatic, renal, and lymphoid cancers, demonstrating broad applicability across tumor types.
Challenges and Considerations
While HRMAS offers numerous advantages, certain challenges must be recognized:
- Heterogeneity Within Tissue: A small core may contain multiple cellular and stromal components; careful sampling and multicore analysis may be required to capture representative metabolic phenotypes.
- Temperature and Handling Effects: Tissue metabolism may change during pre-analysis handling; strict protocols are needed to minimize alterations.
- Spectral Contamination: Biopsy tools or clamps may introduce small contaminants; cleaning protocols and blank controls are advised.
- Standardization and Validation: Differences in sample preparation, spectrometer field strength, and processing can influence results; large-scale studies and protocol standardization are essential before clinical adoption.
Future Directions and Opportunities
- Ultra-High Field and Multinuclear HRMAS: Spectrometers above 800 MHz enable superior resolution and identification of minor metabolites, including 13C and 31P detection from intact tissue cores.
- Integration with Other Imaging Modalities: Combining HRMAS data with MALDI-MS Imaging (MSI) allows spatial mapping of metabolites across tissue sections, linking cellular architecture with molecular content.
- Machine Learning-Driven Analysis: Advanced machine learning and artificial intelligence approaches applied to HRMAS datasets improve classification accuracy, biomarker discovery, and automated pathology-metabolomics integration.
- Multi-Center Clinical Validation: Large, multicenter studies are necessary to validate HRMAS metabolomic markers as diagnostic or prognostic tools across tumor types and patient populations.
- Point-of-Care or Rapid HRMAS Platforms: Development of automated HRMAS workflows compatible with surgical or biopsy suites may enable rapid metabolic readouts to inform intraoperative decision making.
In summary, High-Resolution Magic Angle Spinning NMR represents a robust, non-destructive approach to cancer tissue metabolomics. HRMAS offers a unique advantage in cancer research and diagnosis: molecular insight anchored in morphological context, obtained from minimal, clinically feasible tissue specimens.
Creative Biostructure offers advanced HRMAS NMR services and integrated metabolomic-histopathology workflows tailored for cancer tissue analysis. Our platform enables high-resolution profiling of biopsy or surgical specimens with minimal tissue consumption and maximal informational yield. Contact us today to learn how our expertise in HRMAS NMR can empower your cancer research—from biomarker discovery to tumor phenotyping and translational applications.
References
- Arda Düz S, Mumcu A, Doğan B, et al. Metabolomic analysis of endometrial cancer by high-resolution magic angle spinning NMR spectroscopy. Arch Gynecol Obstet. 2022;306(6):2155-2166.
- Bathen TF, Geurts B, Sitter B, et al. Feasibility of MR metabolomics for immediate analysis of resection margins during breast cancer surgery. Han A, ed. PLoS ONE. 2013;8(4):e61578.
- Giskeødegård GF, Bertilsson H, Selnæs KM, et al. Spermine and citrate as metabolic biomarkers for assessing prostate cancer aggressiveness. Monleon D, ed. PLoS ONE. 2013;8(4):e62375.
- Gogiashvili M, Nowacki J, Hergenröder R, Hengstler JG, Lambert J, Edlund K. HR-MAS NMR based quantitative metabolomics in breast cancer. Metabolites. 2019;9(2):19.
- Paul A, Srivastava S, Roy R, et al. Malignancy prediction among tissues from Oral SCC patients including neck invasions: a 1H HRMAS NMR based metabolomic study. Metabolomics. 2020;16(3):38.
- Penet M, Sharma RK, Bharti S, Mori N, Artemov D, Bhujwalla ZM. Cancer insights from magnetic resonance spectroscopy of cells and excised tumors. NMR in Biomedicine. 2023;36(4):e4724.
- Ruiz-Rodado V, Brender JR, Cherukuri MK, Gilbert MR, Larion M. Magnetic resonance spectroscopy for the study of CNS malignancies. Progress in Nuclear Magnetic Resonance Spectroscopy. 2021;122:23-41.
- Van QN. Current nmr strategies for biomarker discovery. In: Proteomic and Metabolomic Approaches to Biomarker Discovery. Elsevier; 2013:87-117.