Cryo-EM for Bacteriophages
It is crucial that understanding the biological functions of macromolecules by characterizing the molecular structure. As the most effective method to obtain atomic models of proteins and nucleic acids in the 20th century, X-ray crystallography has been fundamental in the development of many scientific fields. But many samples have been proved that it is difficult to crystallize (for example membrane proteins). In addition, the biological macromolecular complexes sometimes cannot be produced in sufficient quantities of crystallization. Cryo-Electron Microscopy (Cryo-EM) has become a potential alternative to X-ray crystallography that has quickly gained popularity in structural biology.
A bacteriophage, also known as a phage, is a virus that infects and replicates within a bacterium. Bacteriophages are omnipresent viruses. With the overuse of antibiotic, the problem of bacterial resistance has become increasingly prominent. Therefor phage therapy has received widespread attention. The first phage therapy publication appearing in 1921 and phage was used to treat the staphylococcus infection in skin. Recently bacteriophage therapy has been widely used for the control of bacterial diseases in humans, for example, phage treatment of infected burns. Thus studying the mechanism of bacteriophage infects bacteria based on the structures of phages will definitely help the development of antibacterial agents and provide an insight into treating human disease. Due to Cryo-EM does not require dehydration of the samples and can produce near atomic-level model, it has been proved that Cryo-EM has become a most convenient and advanced tool for obtaining high-resolution images of bacteriophages. There have many structures of phages obtained by Cryo-EM. For example, it had been reported that the atomic structure of bacteriophage T4 baseplate in its pre- and post-host attachment states obtained by Cryo-EM. Bacteriophage can use the tail to break through the cell surface and get into the interior of cells. With the high-resolution structure of bacteriophage, it helps to explain the events that lead to sheath contraction in atomic detail and understand the mechanism of bacteriophage infects bacteria.
At Creative Biostructure, we have the most advanced high-resolution electron microscopy equipment and a direct electron detector. Our services include the preparation and purification of bacteriophages, image processing of Cryo-EM, and finally we provide our customer with the three-dimensional (3D) reconstruction and near atomic model of bacteriophages. The ultrastructure of bacteriophage can eventually gain an insight into antibacterial research.
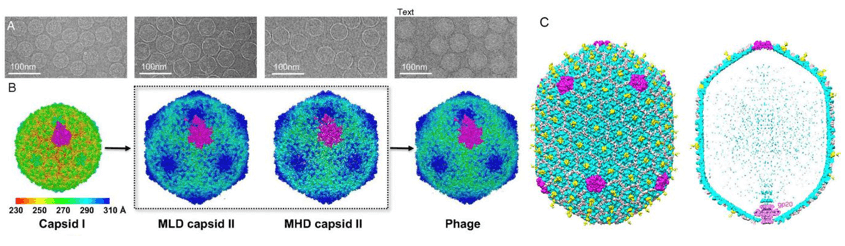 Figure 1. 3D reconstructions of bacteriophages.
Figure 1. 3D reconstructions of bacteriophages.
Creative Biostructure promises to work closely with our customers to provide excellent Cryo-EM strategies for the bacteriophages of your interest.
Please feel free to contact us for a detailed quote.
Ordering Process
References
- Fei Guo, Zheng Liu, et al. Capsid expansion mechanism of bacteriophage T7 revealed by multistate atomic models derived from cryo-EM reconstructions. Proc Natl Acad Sci U S A, 2014; 111:E4606-4614.
- Lei Sun, Xinzheng Zhang, et al. Cryo-EM structure of the bacteriophage T4 portal protein assembly at near-atomic resolution. Nat Commun, 2015; 6:7548.
- Eva Nogales, Sjors H.W. Scheres, Cryo-EM: A Unique Tool for the Visualization of Macromolecular Complexity. Mol Cell, 2015; 58:677-689.
- Stephen T. Abedon, Sarah J. Kuhl, et al. Phage treatment of human infections. Bacteriophage, 2011; 1:66-85.
- Nicholas M. I. Taylor, Nikolai S. Prokhorov, et al. Structure of the T4 baseplate and its function in triggering sheath contraction. Nature, 2016; 533:346-352.

