Glycoprotein Analysis
As an expert in the field of nuclear magnetic resonance, Creative Biostructure provides customers with analysis services of glycoprotein profiling, structure, conformation, and interactions through nuclear magnetic resonance technology.
Problems that We Can Solve
Carbohydrate chains have a significant impact on the physicochemical and biological functional properties of carrier proteins. The thermal stability and solubility of proteins are controlled by carbohydrate chains, which also affect the conformation of proteins, thus controlling their biological functions.
The solution NMR characterization of glycoproteins is the most valuable, because the interactions between carbohydrate molecules and peptides are unexpectedly complex and subtle, often revealing the conformational combination of heterogenetic effects and embedded functional conformations.
Our Technology
NMR spectroscopy is a widely used, non-destructive, atomic resolution and quantifiable analytical technology. Different experiments are used according to the required readings. NMR is most suitable for the structural characterization of glycoproteins. This technology can generate useful information at multiple levels, from the recognition of biomarker signals to the absolute quantification of the whole glycan part of glycoproteins. In addition, NMR has the advantage of studying glycoprotein receptor interactions under physiological conditions.
Service Content
We can express the glycoprotein with a customized label for customers in high yield in eukaryotic culture medium and provide customers with a correct description of glycoprotein structure and dynamics with NMR combined with computing tools.
- Evaluate specific glycosylation patterns and other higher-order structural characteristics
- Composition analysis of proteoglycan content
- Glycoprotein distribution and structure analysis
- Analysis of conformational heterogeneity of a glycoprotein
Production of Isotope Labeled Glycoproteins Through Mammalian Expression Systems
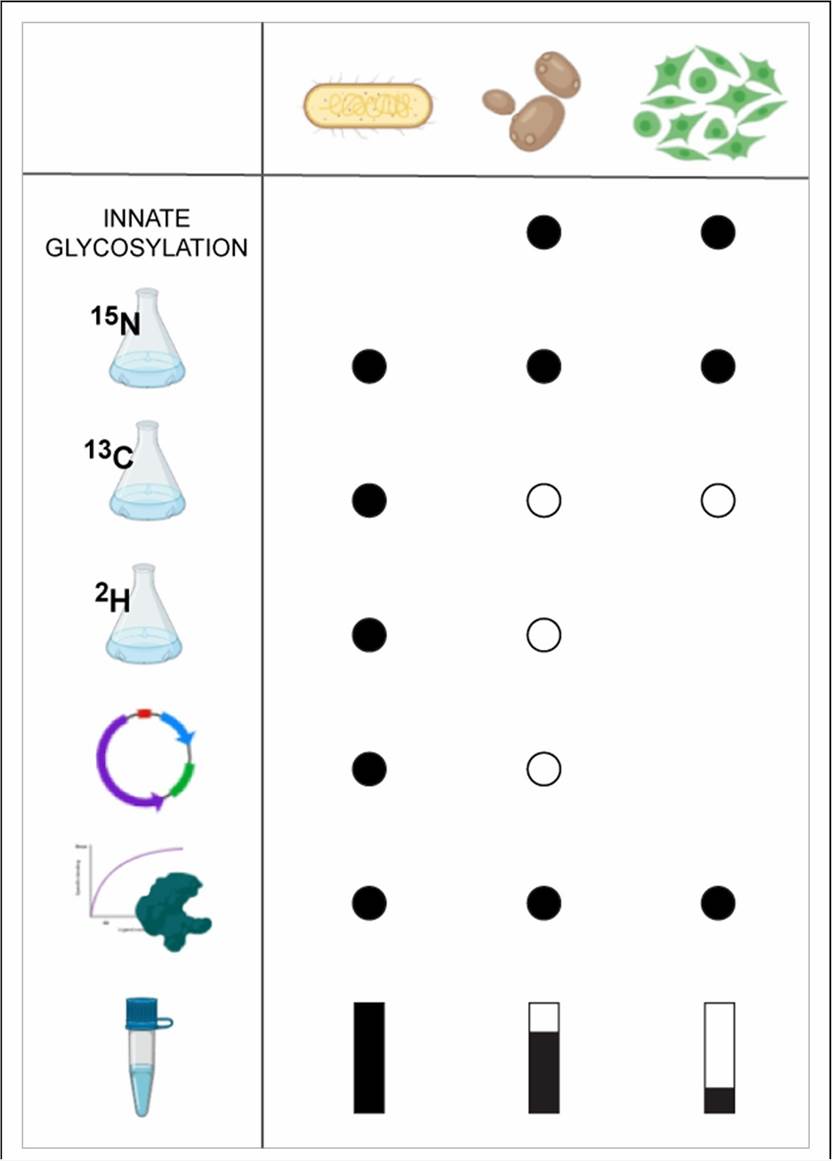 Figure 1. Advantages and limitations of the different systems used in the heterologous expression of glycoproteins (Unione et al., 2021)
Figure 1. Advantages and limitations of the different systems used in the heterologous expression of glycoproteins (Unione et al., 2021)
Because glycoproteins cannot be produced by traditional protein expression systems (such as E. coli), appropriate eukaryotic vectors must be selected to produce glycoproteins with stable isotope-labeled NMR quantities. A typical selection is from mammalian cell lines. We can use mammalian cells to develop metabolic isotope markers of recombinant proteins, including Chinese hamster ovary (CHO) cells, mouse hybridomas and mammalian cells based on adenovirus vectors.
Unified labeling
For uniform 13C/15N labeling of glycoproteins, it is necessary to replace all metabolic precursors with isotopically labeled precursors, which are very expensive commercially. Our method is to use isotope-labeled amino acid mixtures extracted from seaweed and modify the composition of the culture medium.
Partial labeling
Isotopic labeling of the carbohydrate fraction can be easily achieved by using 13C labeled glucose as a metabolic precursor. 13C- and/or 15N-labeled glucosamine (but not N-acetylglucosamine) can be used for metabolic labeling of GlcNAc residues in the carbohydrate portion of glycoproteins.
Other expression systems
In addition to mammalian expression systems, we can also realize isotope labeling through yeast, insect, plant, and other expression systems.
Creative Biostructure is committed to providing high-quality NMR analysis services to advance the life sciences fields. If you have any questions or needs, please contact us and our customer service staff will help you the first time.
Ordering Process
Reference
- Unione L, et al. NMR of glycoproteins: Profiling, structure, conformation and interactions. Current Opinion in Structural Biology. 2021. 68: 9-17.
