Analysis of Peptides in Oriented Biomembranes
Solid state 19F-NMR is a powerful method for studying membrane-active peptides in lipid bilayers under quasi natural conditions. Compared with other nuclei, 19F provides a stronger signal, so it is possible to study the peptide with low concentration (the molar ratio of peptide to lipid is as low as 1:3000), and determine the long distance between the two markers (up to 11Å in the fluid bilayer).
As an expert in the field of nuclear magnetic resonance, Creative Biostructure provides our customers with the analysis services of conformation, direction, dynamics, and aggregation behavior of antibacterial peptides, cell-penetrating peptides and fusion peptides in the membrane through solid-state 19F-NMR analysis.
Advantages of 19F-NMR
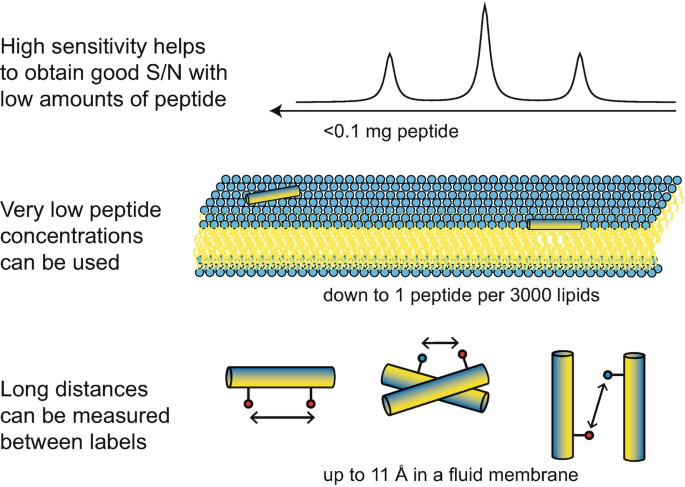 Figure 1. Illustration of the main advantages of solid-state 19F-NMR for studying peptides in membranes (Strandberg & Ulrich, 2018)
Figure 1. Illustration of the main advantages of solid-state 19F-NMR for studying peptides in membranes (Strandberg & Ulrich, 2018)
The main advantage of 19F labeling is the high sensitivity of 19F. Its gyroscope ratio is 94% of 1H, which is the highest among all traditional NMR isotopes.
19F-labeling
In order to study polypeptides with 19F-NMR, they must be specifically labeled with amino acids containing 19F. We achieve this goal through standard peptide synthesis, with a reasonable length of<50 amino acids. To determine the orientation of the peptide in the membrane, the 19F label should be rigidly bound to the peptide skeleton of known geometry.
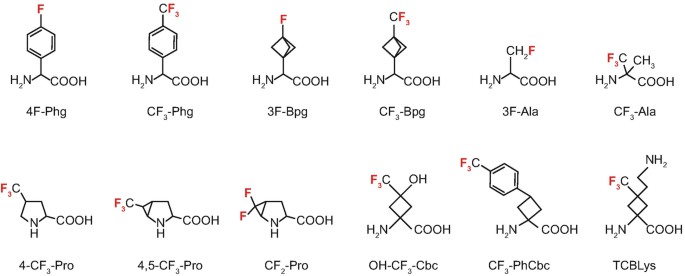 Figure 2. 19F-containing amino acids used for solid-state 19F-NMR structure analysis of membrane-bound peptides (Strandberg & Ulrich, 2018)
Figure 2. 19F-containing amino acids used for solid-state 19F-NMR structure analysis of membrane-bound peptides (Strandberg & Ulrich, 2018)
Determination Service of Peptide Orientation
Our approach
The traditional method of determining the orientation of helical peptides in membranes using 19F-NMR in targeted samples uses NMR data from at least four labeled locations. This involves preparing four different peptides, each with a specific 19F label. Ideally, labels should be evenly distributed around the spiral wheel and should be avoided near the end of the peptide so that all labels are located within the well-folded spiral area. Bipolar coupling (or chemical shift) measured from these locations is used to fit a series of predicted spiral curves. From the best fitting curve, we can get the tilt angle and azimuth angle of the cylindrical peptide, as well as the measurement of its rigid body dynamics.
Detection object
| Type | Examples |
| Fusion Peptides | B18, FP23 |
| Antimicrobial Peptides | PGLa, MSI-103, KIGAKI, SSL-25, Alamethicin, Harzianin, Kalata B1 |
| Cell-Penetrating Peptides | BP100, MAP, TP10 |
Determination Service of Other Types of Membrane-bound Peptides
- Single labels
After a comprehensive analysis based on multiple tags, special simplified cases of standard methods can be used. It is necessary to use characteristic signals that vary significantly with the peptide direction to identify individual marker locations. By focusing on this single label, we can directly and quickly screen the effects of various conditions (such as different lipids and temperatures).
- Distance measurements
When studying membrane-active peptides under quasi natural conditions, 19F-NMR can even determine the distance between markers in fluid membranes. This distance measurement may be essential for determining unusual peptide structures and understanding peptide self-assembly into dimers, pores, or other functional complexes.
Creative Biostructure is committed to providing high-quality NMR analysis services to advance the life sciences fields. If you have any questions or needs, please contact us and our customer service staff will help you the first time.
Ordering Process
Reference
- Strandberg E, Ulrich A S. Solid-state 19F-NMR analysis of peptides in oriented biomembranes. Journal: Modern Magnetic Resonance. 2018. 651-667.
