Characterization of Peptide Structures and Peptide-Lipid Interactions in Membranes
Many important processes in life occur in or around the cell membrane. Lipids have different characteristics in terms of their film-forming ability, fluidity, shape, size, and surface charge. All these factors will affect the way proteins and peptides interact with the membrane. For us to correctly understand these interactions, we need to be able to study all aspects of the interaction between lipids, peptides and proteins.
As an expert in the field of nuclear magnetic resonance, Creative Biostructure relies on our solid-state NMR technology to provide customers with characterization services of peptide structure and peptide-lipid interaction in the membrane. By labeling peptides with different methods, their conformation, orientation, and dynamics in the lipid bilayer can be characterized, and information about their self-assembly and aggregation behavior can be obtained.
Our Technology
Solid-state nuclear magnetic resonance (ssNMR) is an effective method to study the peptide-lipid interaction under quasi-natural conditions. 31P-NMR (from the phospholipid head group) and 2H-NMR (from the deuterated lipid acyl chain) can provide information about the lipid reaction when peptides are added to the membrane. Similarly, 2H -, 13C -, 15N -, and 19F - labels can be selectively incorporated into peptides, so that solid-state NMR experiments can provide direct information about their conformation, orientation, and mobility in the membrane.
Our Services
Estimation of the inclination angle of the α-helix
The most commonly used structural methods are 15N-NMR for the amide-labeled peptide in the main chain and 2H-NMR for the Ala-d3 labeled peptide with CD3 group in the main chain. A single 15N tag can be conveniently used to estimate the tilt angle of the α-helix from the chemical shift of the tag.
Peptides can also be uniformly labeled with 15N. Through the PISEMA two-dimensional experiment, the tilt angle of α-helical peptides can be more accurately analyzed from the generated PISA wheel.
Measurement of azimuth
The amino acid types selective 15N marker can be used to determine the azimuth, which describes the rotation angle around the helix axis, and is particularly important when studying amphoteric peptides.
Evaluation of peptide orientation (tilt and azimuth rotation) and dynamics
Ala-d3 labeled peptides are most likely to be studied by the geometric analysis (GALA) method labeled with alanine, and several positions are labeled one by one, such as alanine scanning. Spiral wave analysis is performed on the segmentation of these positions to determine the direction (tilt angle and azimuth rotation) and dynamics of the whole peptide. 13C-NMR of selectively labeled peptides has also been applied in some studies.
Models for Characterization of Peptide Structures and Peptide-Lipid Interactions in Membranes
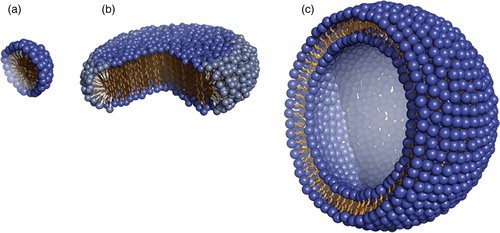 Figure 1. Cartoon model of three membrane mimetic media. (a) A detergent micelle, (b) a two-component bicelle and (c) a lipid vesicle (Mäler, 2012)
Figure 1. Cartoon model of three membrane mimetic media. (a) A detergent micelle, (b) a two-component bicelle and (c) a lipid vesicle (Mäler, 2012)
A typical biofilm is a complex structure, mainly composed of lipids and proteins. To study the molecular details in the structure of peptides or proteins by NMR, we need to use a two-layer correlation model. The natural biofilm is very complex, and the preparation from cells is also very cumbersome. Therefore, model membranes composed of synthetic lipids or lipid mixtures have been used for most biophysical studies.
We use a wide range of lipids with different head groups, chain lengths, and chain saturation, as well as liposoluble and branched lipids, to prepare model membranes with different properties. This method makes it possible for us to systematically evaluate the effects of different lipid properties on membrane binding-peptides for customers.
Creative Biostructure is committed to providing high-quality NMR analysis services to advance the life sciences fields. If you have any questions or needs, please contact us and our customer service staff will help you the first time.
Ordering Process
Reference
- Mäler L. Solution NMR studies of peptide-lipid interactions in model membranes. Molecular Membrane Biology. 2012, 29(5): 155-176.
