Antiviral Research
Viruses, as one of the types of pathogens, can bring diseases to their host. In particular, some viruses cause infectious diseases and impact global health in both animals and humans, such as coronavirus (SARS-CoV, MERS-CoV, and SARS-CoV-2). Some evidence suggests that other serious human diseases such as diabetes, rheumatoid arthritis, immunologic disorders, and some tumors can also be caused by viruses. Studies on viral infection, replication, assembly, virion release, and the interaction with host cells help us to understand viral pathogenesis and accelerate the development of antiviral drugs. Structural virology is focused on the research of the molecular mechanism of viruses invading to host cells, release, and immune evasion, which is of great significance for virus-induced disease prevention and control as well as related drug design. Traditional structural analysis methods include X-ray crystallography and nuclear magnetic resonance (NMR), but virus particles usually have huge molecular weight and are difficult to be crystallized. NMR is only suitable for small biomolecular. However, cryogenic electron microscopy (cryo-EM) overcomes these problems without crystallizing the sample. In addition, virus particles generally have high symmetry, which can be used in cryo-EM to reduce image noise and improve the resolution of the final model in the process of data analysis. A large number of virus structures have been analyzed by cryo-EM and promoted antiviral drug discovery.
Recently, significant progress has been made in the structural analysis of COVID-19 (SARS-CoV-2). With the revealing of SARS-CoV-2 spike protein structure, scientists found that a neutralizing human antibody (4A8) binds to the N-terminal domain (NTD) of the Spike protein of SARS-CoV-2. Combination with neutralizing antibody 4A8 and RBD-targeted antibodies may be a promising therapy to avoid the escape of the virus. These studies can be used in the development of structure-based vaccine design against SARS-CoV-2.
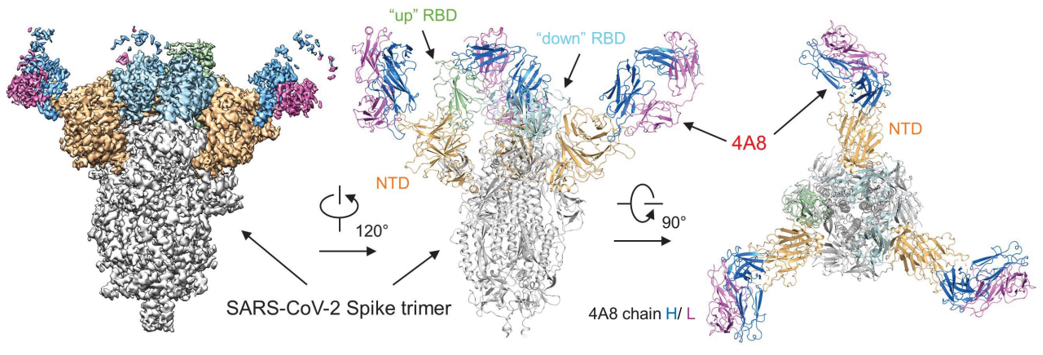 Figure 1. Cryo-EM structure of the 4A8 and S-ECD complex (Chi X, et al. 2020)
Figure 1. Cryo-EM structure of the 4A8 and S-ECD complex (Chi X, et al. 2020)
Adeno associated virus (AAV), belonging to the dependent virus genus of the family Parvoviridae, is the simplest non-enveloped single-stranded DNA defective virus known at present. It is considered to be the most promising gene therapy vector and is also widely used in the study of gene therapy in vivo. Scientists used the particle filter algorithm to analyze the high-resolution cryo-EM structure of the AAV2/ AAVR complex, with a resolution of 2.8 Å. The key binding points of AAV2 and AAVR were determined, which provided high-resolution details of AAV2 entering cells. This study provides a high-resolution structural basis for an in-depth understanding of the biological characteristics of AAV and helps us to understand its molecular mechanism of infecting the host. Besides, it is also conducive to the further development of AAV vectors for gene therapy.
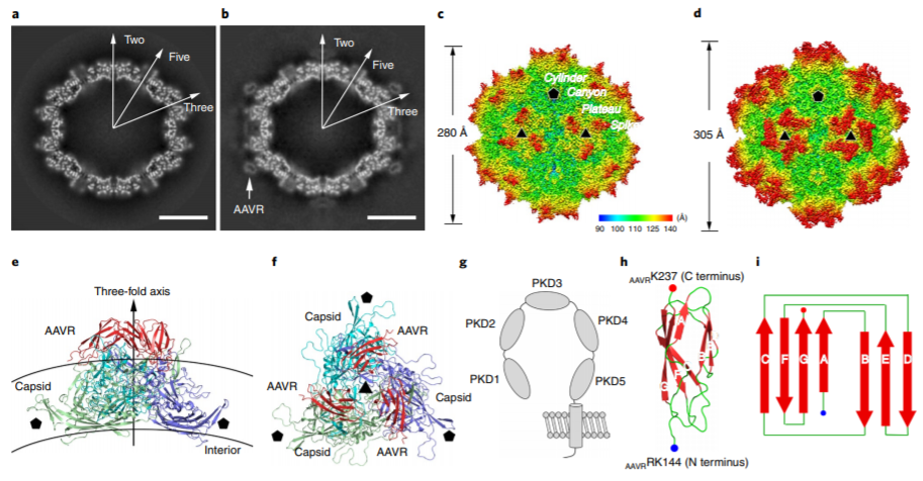 Figure 2. The cryo-EM structures of the AAV2 / AAVR complex (Zhang R, et al. 2019)
Figure 2. The cryo-EM structures of the AAV2 / AAVR complex (Zhang R, et al. 2019)
Human cytomegalovirus (HCMV), which belongs to the Herpesviridae β Subfamily, is a kind of double-stranded DNA envelope virus. It is widely spread in humans, which can cause a variety of diseases. The nucleocapsid, which is responsible for genome packaging and transport, has become an important target for the development of new herpesvirus drugs. Another team used cryo-EM to analyze the first β in situ portal structure of high-resolution portal and capsid vertex specific components (CVSC) of subfamily herpesvirus HCMV. It provides a new idea for understanding the molecular mechanism of pressure sensing and regulation in the process of herpesvirus genome packaging, stabilization, and release.
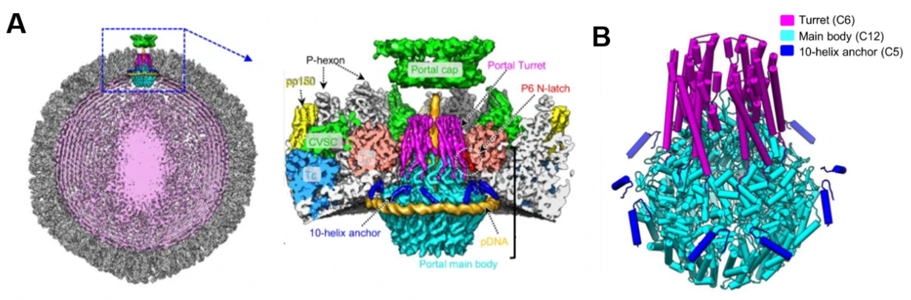 Figure 3. Cryo-EM structure of HCMV nucleocapsid and HCMV portal apex, HCMV portal in situ structure model (B) (Li Z, et al. 2021)
Figure 3. Cryo-EM structure of HCMV nucleocapsid and HCMV portal apex, HCMV portal in situ structure model (B) (Li Z, et al. 2021)
With our cryo-EM platform, Creative Biostructure can provide sample preparation and data collection as well as analysis services. If you are interested in our services, please feel free to contact us. We are looking forward to cooperating with you.
Ordering process
References
- Chi X, et al. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science. 2020, 369(6504): 650-655.
- Zhang R, et al. Adeno-associated virus 2 bound to its cellular receptor AAVR. Nature Microbiology. 2019, 4(4): 675-682.
- Li Z, et al. Structural basis for genome packaging, retention, and ejection in human cytomegalovirus. Nature Communications. 2021, 12(1): 1-14.