Nuclear Magnetic Resonance (NMR) spectroscopy is a gold-standard analytical tool used across chemistry, biochemistry, and pharmaceutical science for structural and dynamic characterization of molecules. However, its inherent low sensitivity often necessitates large sample volumes (>200 μL) and high concentrations (>0.5 mM for proteins), leading to long acquisition times that limit applicability with mass-limited or dilute samples. Advances like cryogenic probe technology have addressed this bottleneck, enabling high-quality data collection from samples at micromolar concentrations and sub-nanomole quantities.
This article explores the principles, implementation, benefits, constraints, and real-world applications of cryogenic NMR probes.
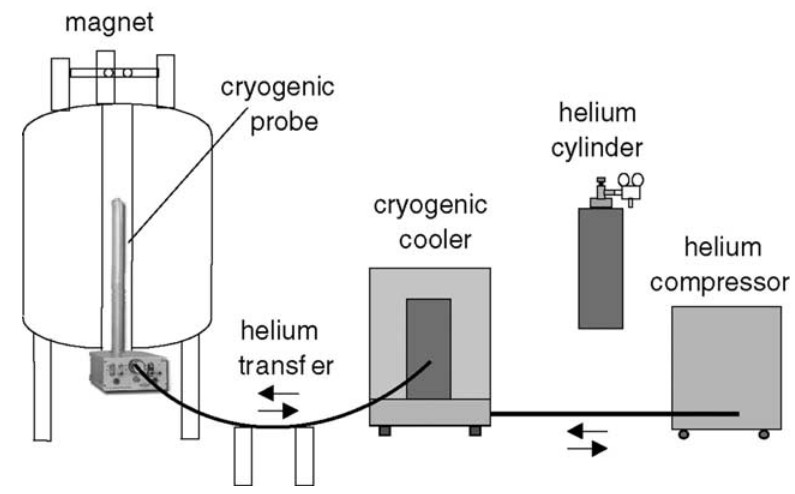 Figure 1. Schematic presentation of the closed-loop cryogenic cooling system consisting of the cooling equipment, helium compressor and the cryogenic probe. (Kovacs et al., 2005)
Figure 1. Schematic presentation of the closed-loop cryogenic cooling system consisting of the cooling equipment, helium compressor and the cryogenic probe. (Kovacs et al., 2005)
Why Sensitivity Matters for Low-Concentration Samples
Low-concentration samples-such as fragment library compounds, precious biotechnology targets, dilute biofluids, or novel natural products-pose a critical challenge: classical NMR sensitivity often requires milligram quantities or high micromolar concentration, necessitating long experiment times and large sample volumes. Cryogenic NMR probes fundamentally transform this paradigm by delivering dramatic signal-to-noise ratio (SNR) enhancement, allowing:
- Structural characterization of proteins at <<100 µM, enabling experiments like TROSY, chemical shift mapping, and ligand binding.
- Fragment-based screening with weak binders at low micromolar concentration.
- Metabolite detection in biofluids where concentrations may be nanomolar to low micromolar.
- Structural elucidation of natural products from microgram quantities using 13C/HSQC experiments.
In industrial R&D-whether pharma, biotech, or academia-cryogenic probes provide accelerated throughput, reduced material consumption, and reliable insight into low-abundance analytes.
Principles Behind Cryogenic Probe Sensitivity Enhancement
Cryogenic probes boost SNR by addressing key sources of electronic and thermal noise and optimizing RF detection efficiency. The four principal mechanisms are:
Reduced Thermal Noise
Thermal (Johnson-Nyquist) noise generated in detection coils and electronics is proportional to the square root of temperature. Conventional probes operate at ~300 K, whereas cryogenic probes cool coils and electronics to ~20-30 K. This reduces thermal noise by √(300/20) ≈ 3.9×. Empirical improvements typically reach 3-5× SNR over room-temperature probes. Reduced electronic noise means that even weak NMR signals from dilute samples become detectable with high fidelity.
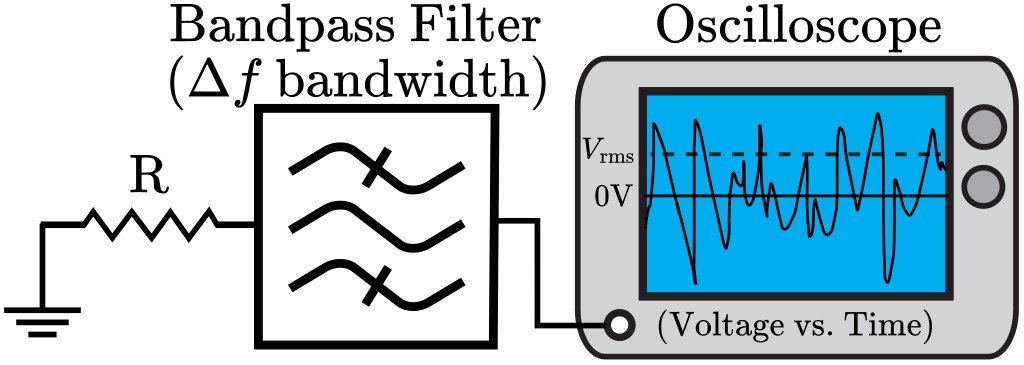 Figure 2. Schema of Johnson-Nyquist noise.
Figure 2. Schema of Johnson-Nyquist noise.
Increased Coil Quality Factor (Q)
At low temperatures, the resistivity of conductive materials (e.g. copper, silver) drops dramatically. Q-a ratio of stored energy to dissipated energy-is inversely proportional to resistance. Lower resistance means higher Q, which enhances sensitivity, narrows coil bandwidth, and improves signal storage efficiency. This effect is especially valuable for dilute samples or heteronuclei (13C, 15N), as the coil becomes more efficient at capturing faint signals.
Cooled Low-Noise Amplifiers
Cryogenic cooling extends beyond coils to include the preamplifiers-often GaAs FETs or HEMT devices. Cooling these electronic components sharply reduces their noise figure and shot noise, preserving the weak NMR signal before it reaches room-temperature electronics. The combined reduction in coil and amplifier noise results in system SNR improvements of 3× to 5×, making previously undetectable spectra accessible.
Cryocooler Technologies
Cryogenic probes utilize either closed-cycle helium cooling systems or open-cycle liquid nitrogen/helium setups to maintain cryogenic temperatures:
- Closed-Cycle Helium Systems: Self-contained, long-duration operation (~4 h cool-down), minimal maintenance, environmentally sustainable.
- Liquid-Nitrogen (Prodigy-Style): Faster start-up (~2 h cool-down), ease of installation, and still realize 2-3× sensitivity boosts compared to room-temperature probes.
These systems thermally isolate the sample (kept at ambient or controlled temperature) from the cold detection circuit, preserving spectral integrity while enabling high sensitivity.
Quantitative Gains from Cryogenic Probe Implementation
1D Proton (1H) NMR
In non-polar organic solvents, cryogenic probes deliver an SNR improvement of ~3-4× relative to room-temperature probes. In aqueous/buffered biological samples, even with conductive losses, typical gains are ~2×-2.5×. These SNR improvements translate to accelerated acquisitions:
- 3× gain → ~9× faster experiments
- 4× gain → ~16× faster or equivalent quality in fewer scans
For high-throughput workflows-screening, metabolomics, fragment-based assays-time savings are tremendous.
Carbon-13 (13C) and Heteronuclei NMR
13C experiments suffer from low natural abundance and sensitivity. Cryogenic probes yield:
- ~7× higher SNR per scan
- Up to ~11× better time-normalized sensitivity compared to conventional probes
This capability allows acquisition of high-quality 13C spectra from microgram samples or low-millimolar concentration, supporting structure elucidation, impurity profiling, and metabolite quantification.
Salt and Buffer Conductivity Effects
High ionic strength buffers increase dielectric losses, introducing sample noise and marginally reducing SNR gain. However, cryogenic probes still deliver substantial advantages-typically ~2×-3× SNR improvement even in high-conductivity samples. Mitigating strategies, such as using low-conductivity deuterated buffers or reduced salt concentration, can preserve maximal gain.
Advanced designs also adapt to smaller bore diameters (e.g., 3 mm tubes) to limit sample noise, while preserving filling factor and sensitivity.
Application-Oriented Advantages
Cryogenic probes enhance NMR utility across key industrial and translational domains.
Structural Biology & Protein NMR
Cryogenic triple-resonance probes (e.g., TCI or HCN configurations) play a critical role in enabling high-resolution NMR spectroscopy on biomacromolecules at low micromolar concentrations, typically in the 10-50 µM range. These probes have made it feasible to acquire high-quality spectra for proteins and protein complexes that are otherwise challenging due to size, solubility, or dynamic behavior.
Key capabilities include:
- Acquisition of advanced multidimensional experiments such as 2D and 3D TROSY, HSQC, HNCO, and HNCA, even for large proteins or multi-domain assemblies.
- Measurement of dynamic processes through relaxation dispersion, chemical exchange, and titration studies in low-concentration regimes.
- Detection and quantification of protein-ligand interactions in fragment-based screening or lead validation studies.
The enhanced sensitivity provided by cryogenic probes allows structural investigations of proteins and complexes that were previously impractical using conventional probes, thereby facilitating progress in structure-based drug design and molecular biophysics.
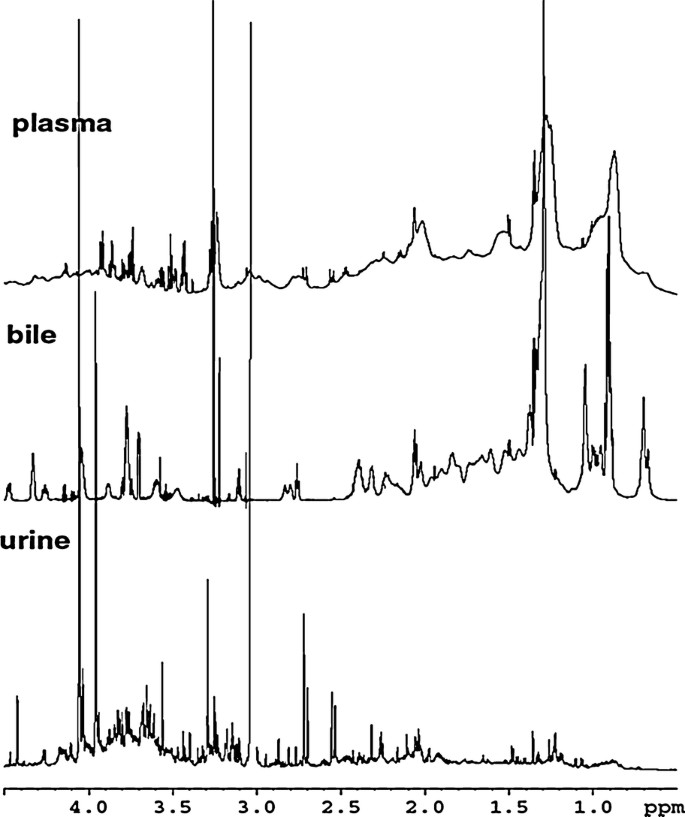
Metabolomics & Biofluid Profiling
In metabolomics and clinical biomarker discovery, the detection of metabolites at low micromolar to nanomolar concentrations within complex biological matrices poses a major analytical challenge. Cryogenic probes address this challenge by delivering significant gains in signal-to-noise ratio, which translates directly into increased detection sensitivity and quantitative accuracy.
Specific benefits include:
- High-throughput acquisition of 1D 1H and 2D 13C spectra from small volumes of biofluids such as urine, plasma, and cerebrospinal fluid.
- Reliable quantification of comprehensive metabolite panels in both preclinical and clinical studies.
- Enhanced capability to detect low-abundance biomarkers in complex or variable sample environments.
These features make cryogenic NMR probes particularly valuable for longitudinal disease monitoring, metabolic pathway analysis, and personalized medicine initiatives.
Fragment and Drug Screening
Fragment-based drug discovery (FBDD) often relies on the ability to detect weak, transient interactions between low-molecular-weight ligands and target proteins. The inherently low affinity of these interactions typically requires high concentrations of both protein and ligand to achieve measurable binding-conditions that can be prohibitive without the enhanced sensitivity of cryogenic probes.
Cryogenic NMR enables:
- Detection of fragment binding events at ligand concentrations of 100 µM or lower.
- Substantial reduction in acquisition times per sample, facilitating rapid screening of large compound libraries.
- Seamless integration with automated liquid-handling systems for high-throughput workflows.
Recent advancements, such as the combination of cryogenic probes with hyperpolarization techniques (e.g., photo-CIDNP), have further enhanced sensitivity by factors of 20-100, enabling the detection of even weaker interactions and smaller fragments in early-stage screening campaigns.
Natural Products Chemistry
In the field of natural product discovery, researchers frequently work with microgram quantities of isolated compounds. Cryogenic NMR probes are particularly advantageous in this context, as they allow meaningful structural elucidation with minimal sample consumption.
They support:
- Acquisition of broadband 13C and 2D correlation spectra (e.g., HSQC, TOCSY, HMBC) from sub-milligram quantities of sample.
- Structural characterization without the need for extensive purification or sample concentration, thereby accelerating the workflow.
- Sensitive impurity profiling during initial screening stages, which supports dereplication and compound prioritization efforts.
These capabilities are essential for natural product chemists and synthetic organic chemists involved in lead identification and early-stage development, where sample limitations are common.
Solid-State & MAS NMR (CryoMAS Probes)
The application of cryogenic technology has extended beyond solution-state NMR into solid-state environments through the development of cryogenically cooled magic-angle spinning (CryoMAS) probes. These innovations have significantly improved the utility of solid-state NMR spectroscopy for challenging samples.
Notable advantages include:
- Up to ten-fold improvement in signal-to-noise ratio, with no requirement for isotopic enrichment or alteration of the sample composition.
- Enhanced sensitivity in structural studies of amyloid fibrils, membrane proteins, polymeric solids, and other low-mobility systems.
- Ability to obtain high-resolution spectra from low-concentration or limited-quantity samples, thereby reducing experiment durations and sample requirements.
CryoMAS probes have proven transformative for structural investigations in pharmaceutical formulation science, materials characterization, and structural virology.
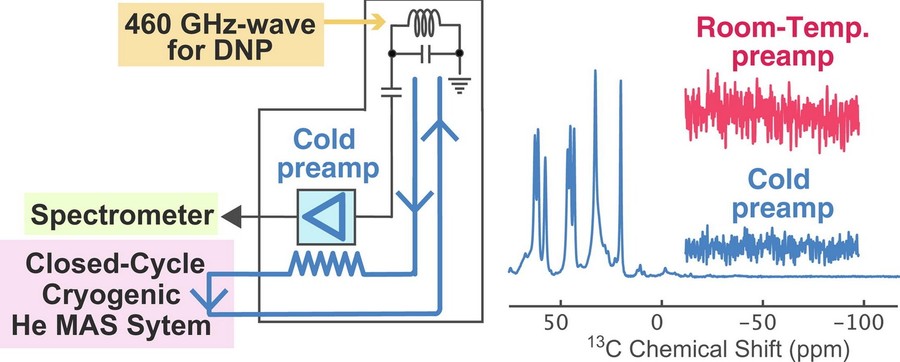 Figure 3. Cryogenic signal amplification combined with helium-temperature MAS DNP. (Matsuki et al., 2022)
Figure 3. Cryogenic signal amplification combined with helium-temperature MAS DNP. (Matsuki et al., 2022)
Emerging Trends and Future Directions
Cryogenic probe technology continues to evolve in several exciting directions:
- Integration with Hyperpolarization Techniques: Combining cryo-probes with photo-CIDNP or DNP methods enables dramatic boosts (20×-100×) in SNR and expands reach to ultra-low concentrations in seconds.
- Microcoil Cryo-Hybrid Probes: These marry cryocooling with microcoil architectures to allow detection of picomole analytes (picoliter flows), enabling next-generation fragment screening and single-cell metabolomics.
- Ultra-high Field & CryoMAS: Pushing field strength to 1 GHz+ yields further intrinsic sensitivity gains, while cryogenic MAS probes open new possibilities in solid-state structural biology (e.g., protein fibrils, membrane assemblies).
Select Service
Case Studies
Case 1: Contribution of protein conformational heterogeneity to NMR line shapes at cryogenic temperatures
This study explores the cause of spectral broadening in low-temperature NMR of proteins, focusing on conformational heterogeneity as a contributing factor. Using Escherichia coli dihydrofolate reductase (DHFR) as a model, researchers measured backbone torsion angles (Ψ) at 105 K to investigate whether multiple conformations, frozen from their room-temperature equilibrium states, contribute to broad linewidths.
By selectively enriching isoleucine residues with 15N and 13C and applying a modified NCCN Ψ experiment, they identified three distinct conformational states for the I60-I61 amide pair. These states corresponded to Ψ angles of 114°, 150°, and 164°, compared to 118° observed in crystal structures. The results confirm that frozen conformational distributions at cryogenic temperatures can lead to inhomogeneous broadening in protein NMR spectra, offering key insights into the interpretation of low-temperature NMR data.
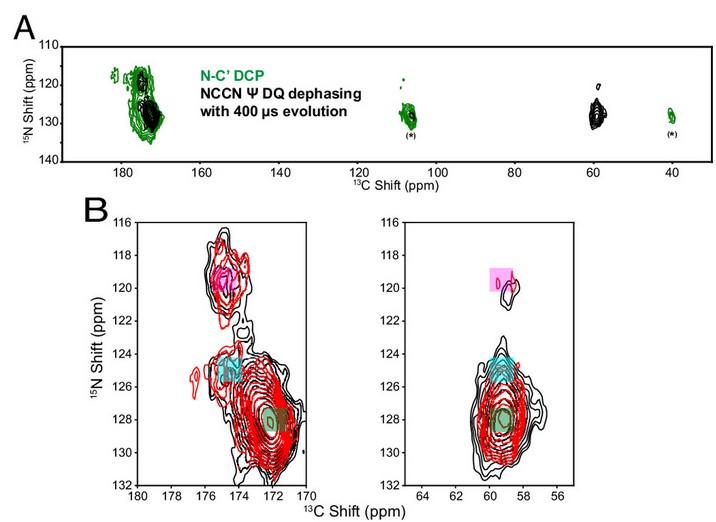 Figure 4. DNP spectra of 13C,15N-Ile DHFR:TMP (I-DHFR) collected at 105 K. (Yi et al., 2024)
Figure 4. DNP spectra of 13C,15N-Ile DHFR:TMP (I-DHFR) collected at 105 K. (Yi et al., 2024)
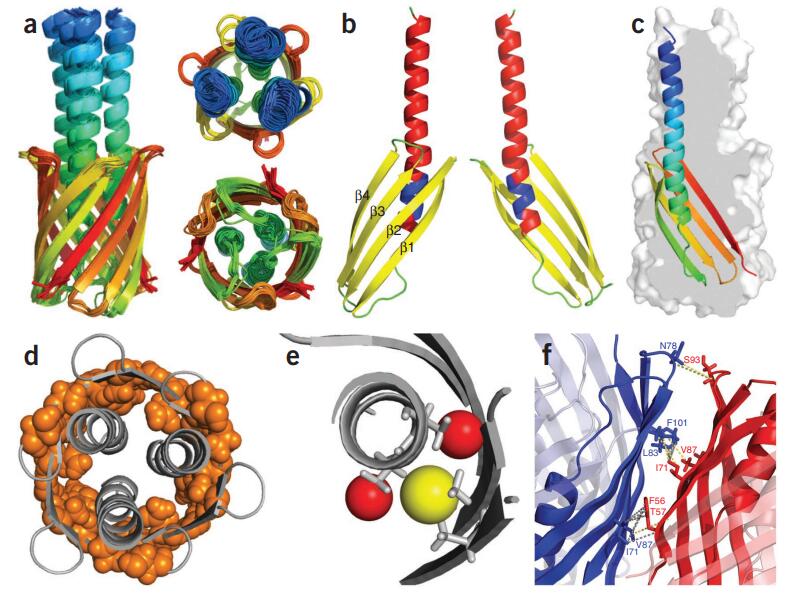
Case 2: Solid-state NMR MAS CryoProbe enables structural studies of human blood protein vitronectin bound to hydroxyapatite
This study highlights how cryogenically cooled probe technology significantly enhances the sensitivity of solid-state NMR, overcoming key limitations in analyzing complex biomolecular assemblies. Using a CryoProbe optimized for magic angle spinning (MAS), researchers investigated the structure of the human blood protein vitronectin (Vn) bound to hydroxyapatite (HAP), a mineral form of calcium phosphate implicated in various diseases.
The enhanced sensitivity of the CryoProbe enabled acquisition of high-resolution 3D NMR spectra, allowing for sequential residue assignments and site-specific characterization of water-protein interactions within the Vn-HAP complex. These findings offer valuable atomic-level insights into how Vn associates with HAP, a process relevant to pathological conditions such as macular degeneration and Alzheimer's disease, thereby advancing the structural understanding of these disease-related biomineralization events.
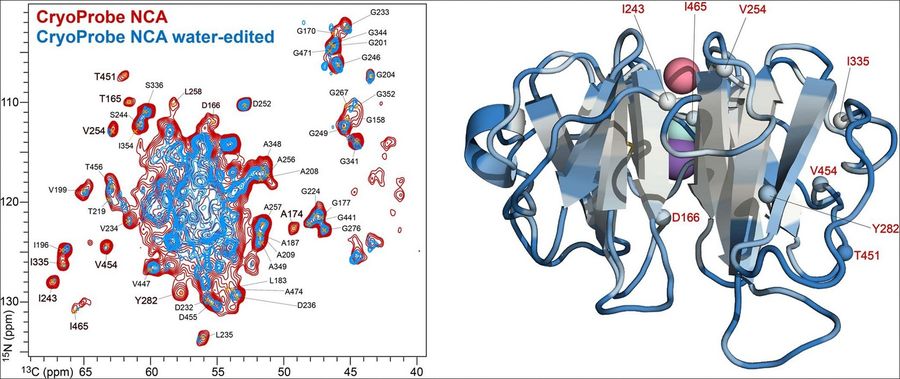 Figure 5. Structural insights into the vitronectin-hydroxyapatite assembly were revealed using the CryoProbe. (Gopinath et al., 2024)
Figure 5. Structural insights into the vitronectin-hydroxyapatite assembly were revealed using the CryoProbe. (Gopinath et al., 2024)
In summary, cryogenic NMR probes represent a transformative technology for low-concentration NMR spectroscopy. By dramatically reducing thermal and electronic noise, these hardware solutions enable analyses previously considered impossible-protein complexes at low micromolar regimes, rapid 13C profiling of biofluids, microliter-scale flows, and beyond. While not a panacea-some sensitivity loss remains with conductive buffers-their unmatched gains in sensitivity (3-5× or higher) and time savings (up to ~16× faster) position cryo-probes as essential tools in modern analytical and structural research. With continued advances in microcoils, DNP, and quantum sensing technologies, cryogenic probes are poised to remain at the vanguard of ultra-sensitive NMR detection.
Creative Biostructure provides cutting-edge NMR services and tailored structural analysis solutions to support your research and industrial needs. Contact us today to explore how our expertise can accelerate your discovery.
References
- Gopinath T, Shin K, Tian Y, et al. Solid-state NMR MAS CryoProbe enables structural studies of human blood protein vitronectin bound to hydroxyapatite. Journal of Structural Biology. 2024;216(1):108061.
- Kovacs H, Moskau D, Spraul M. Cryogenically cooled probes-a leap in NMR technology. Progress in Nuclear Magnetic Resonance Spectroscopy. 2005;46(2-3):131-155.
- Matsuki Y, Nakamura S, Hobo F, et al. Cryogenic signal amplification combined with helium-temperature MAS DNP toward ultimate NMR sensitivity at high field conditions. Journal of Magnetic Resonance. 2022;335:107139.
- Yi X, Fritzsching KJ, Rogawski R, Xu Y, McDermott AE. Contribution of protein conformational heterogeneity to NMR lineshapes at cryogenic temperatures. Proc Natl Acad Sci USA. 2024;121(8):e2301053120.