The biopharmaceutical industry has seen exponential growth in recent years, with biologics such as monoclonal antibodies, vaccines, recombinant proteins, and gene therapies revolutionizing modern medicine. With this growth comes the critical need for robust and precise quality control (QC) methodologies to ensure product consistency, safety, and efficacy. Nuclear Magnetic Resonance (NMR) spectroscopy has emerged as a powerful tool in this arena, offering a non-destructive, reproducible, and structurally informative technique for comprehensive molecular characterization. NMR fingerprinting, in particular, plays a pivotal role in the quality assessment of complex biologics.
Creative Biostructure offers comprehensive NMR analysis services tailored to the quality control needs of the biopharmaceutical industry. This article presents an in-depth overview of NMR fingerprinting as a powerful tool for quality assessment in biopharmaceuticals, covering its underlying principles, key applications, benefits, current challenges, and regulatory perspectives.
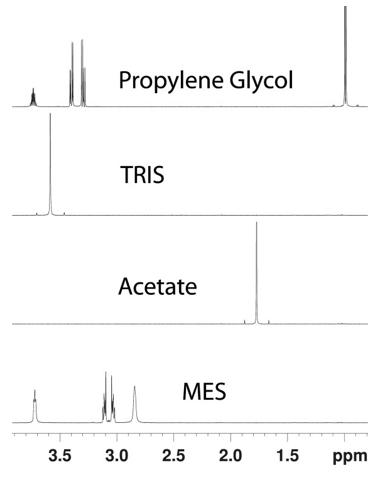
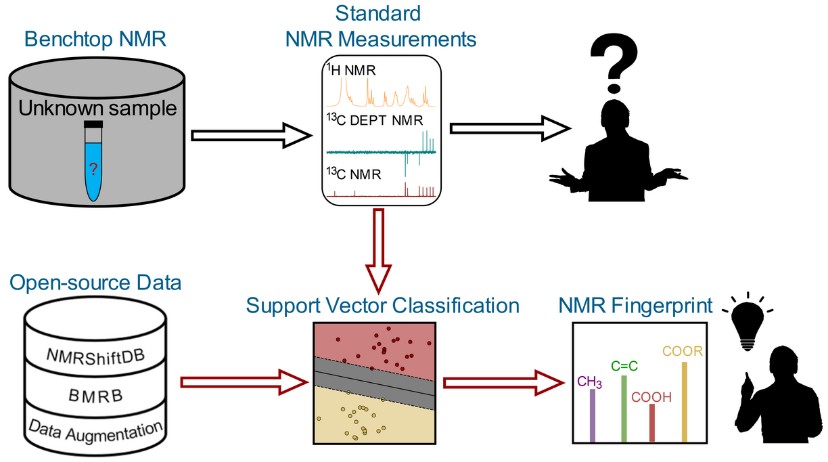 Figure 1. NMR fingerprinting tool. (Specht et al., 2024)
Figure 1. NMR fingerprinting tool. (Specht et al., 2024)
Principles of NMR Spectroscopy
NMR spectroscopy is based on the interaction of atomic nuclei with an external magnetic field. Certain nuclei, such as 1H, 13C, 15N, and 31P, possess spin properties that allow them to absorb and re-emit radiofrequency energy when placed in a magnetic field. The resonance frequency of a nucleus is influenced by its chemical environment, producing a unique spectral pattern—its "fingerprint."
Key NMR concepts include:
- Chemical Shift: Indicates the electronic environment surrounding a nucleus.
- Coupling Constants: Provide information about the number and types of neighboring nuclei.
- Signal Intensity: Proportional to the number of nuclei contributing to a particular resonance.
- Relaxation Times (T1 and T2): Describe the return of nuclear spin populations to equilibrium.
Advanced NMR methods, such as two-dimensional (2D) NMR (e.g., COSY, HSQC, TOCSY), enable detailed structural elucidation by correlating signals between different nuclei.
What is NMR Fingerprinting?
NMR fingerprinting is a powerful analytical approach used to capture the unique structural and compositional characteristics of biopharmaceutical products. Much like a molecular barcode, an NMR fingerprint consists of a spectral pattern that is highly specific, reproducible, and reflective of a product's chemical identity and integrity. This technique enables detailed batch-to-batch comparison, monitoring of manufacturing consistency, and the detection of subtle structural differences that may arise from formulation changes, storage conditions, or process variations.
By providing atomic-level resolution, NMR fingerprinting facilitates a holistic assessment of complex biological products such as monoclonal antibodies, recombinant proteins, vaccines, and other biologics. Because NMR is non-destructive and does not require prior separation or labeling, it offers a direct and unbiased view of the sample, making it an invaluable tool for both quality control and comparability studies in biopharmaceutical development and production.
Types of NMR Fingerprints
- One-Dimensional (1D) 1H NMR Spectra: This is the most commonly used NMR fingerprinting method. The 1H NMR spectrum provides a rapid, global overview of the sample's hydrogen-containing molecular features, capturing the chemical environments of protons throughout the molecule. It is particularly effective for routine batch verification, detection of excipient variations, and overall formulation assessment.
- Two-Dimensional (2D) NMR Spectra (e.g., 1H-13C HSQC): Two-dimensional techniques like Heteronuclear Single Quantum Coherence (HSQC) spectroscopy significantly enhance resolution and sensitivity for larger and more complex biomolecules. These spectra allow for the correlation of proton and carbon nuclei within a molecule, providing a detailed map of the molecular structure. This is especially useful for evaluating higher-order structures, post-translational modifications (e.g., glycosylation), and minor structural variants that may not be evident in 1D spectra.
- Metabolite Fingerprints: In the context of bioprocess monitoring, NMR metabolomics can be applied to obtain metabolic fingerprints of host cells (e.g., CHO cells) or fermentation broths. These spectra reveal real-time insights into cell viability, nutrient consumption, waste accumulation, and potential contamination, offering a systems-level view of production health. This is crucial for ensuring consistent product quality during upstream processing.
Applications of NMR Fingerprinting in Biopharmaceutical QC
Structural Confirmation
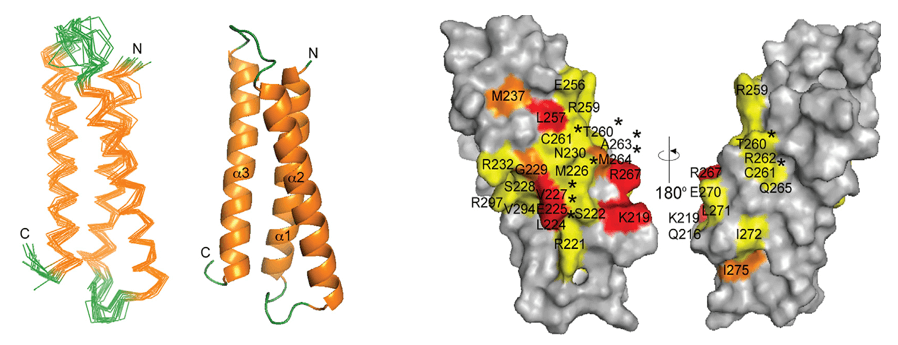
One of the most fundamental applications of NMR fingerprinting is the structural confirmation of biopharmaceutical products. Nuclear magnetic resonance provides a detailed molecular-level view of the primary amino acid sequence (primary structure), as well as the protein's folding pattern (secondary and tertiary structures). Techniques such as 2D 1H-15N HSQC are particularly valuable for characterizing therapeutic proteins and monoclonal antibodies. These spectra act as a unique "fingerprint" of the protein, enabling the identification of conformational changes due to manufacturing variations, formulation conditions, or degradation over time. Even minor shifts in peak positions can indicate subtle alterations in folding, post-translational modifications, or aggregation—each of which can critically impact biological function and efficacy.
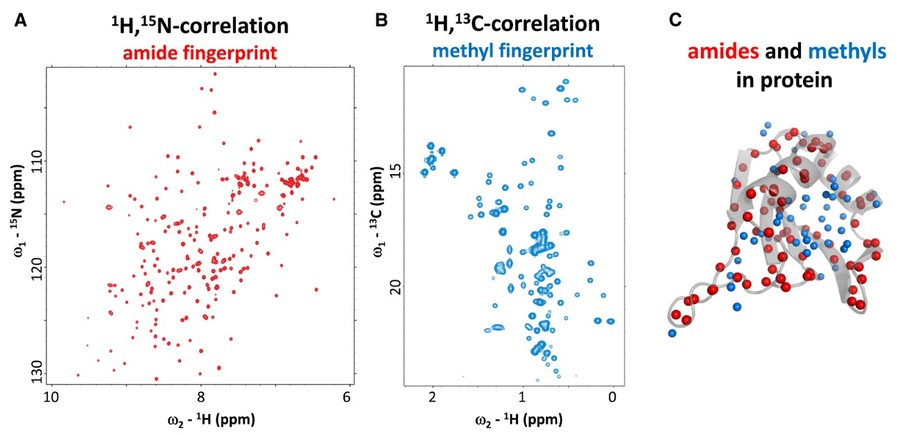 Figure 2. NMR fingerprint spectra for amides and methyls. (A) 1H-15N (red) and (B) 1H-13C (cyan) correlation NMR spectra showing amide and methyl NMR signals. (C) Amide and methyl groups (red and cyan spheres, respectively) are well distributed in a protein structure providing unique probes to characterize the structure and dynamics of proteins and protein complexes. (Kang and Sattler, 2018)
Figure 2. NMR fingerprint spectra for amides and methyls. (A) 1H-15N (red) and (B) 1H-13C (cyan) correlation NMR spectra showing amide and methyl NMR signals. (C) Amide and methyl groups (red and cyan spheres, respectively) are well distributed in a protein structure providing unique probes to characterize the structure and dynamics of proteins and protein complexes. (Kang and Sattler, 2018)
Batch-to-Batch Consistency
Ensuring consistency across multiple manufacturing batches is essential for regulatory compliance and therapeutic reliability. NMR fingerprinting offers a robust method for comparing the spectral profiles of different production lots. A high degree of spectral overlap indicates consistent product quality, while any deviation—whether in peak shape, chemical shift, or intensity—can flag potential issues such as contamination, process drift, or product degradation. Unlike some analytical methods that focus solely on specific attributes, NMR evaluates the sample as a whole, making it an ideal orthogonal tool for comprehensive batch assessment.
Detection of Impurities and Degradation Products
Biopharmaceuticals are inherently sensitive to various degradation pathways, including oxidation, deamidation, glycation, and hydrolysis. NMR spectroscopy excels at identifying and quantifying such chemical modifications. Its ability to observe intact molecules without prior separation enables the detection of both known and unknown impurities. For example, the emergence of new peaks or the disappearance of established ones in 1H NMR spectra can signify the formation of degradation products or interactions with excipients. In the case of long-term stability studies or accelerated degradation testing, NMR serves as an essential tool to monitor structural integrity over time.
 Figure 3. Pharmaceutical impurities and degradation products: Uses and applications of NMR techniques. (Maggio et al., 2014)
Figure 3. Pharmaceutical impurities and degradation products: Uses and applications of NMR techniques. (Maggio et al., 2014)
Biosimilar Comparability
In the regulatory landscape for biosimilars, demonstrating analytical similarity to an originator biologic is a fundamental requirement. NMR fingerprinting is increasingly recognized by agencies such as the FDA and EMA as a key component of the analytical comparability toolkit. When used in conjunction with orthogonal methods like mass spectrometry and high-performance liquid chromatography (HPLC), NMR contributes valuable insights into higher-order structure and conformational dynamics. Comparative HSQC spectra, for example, can reveal differences in folding or domain flexibility that may not be detectable by mass-based techniques alone. Thus, NMR supports the scientific justification for biosimilarity, aiding in regulatory approval and market entry.
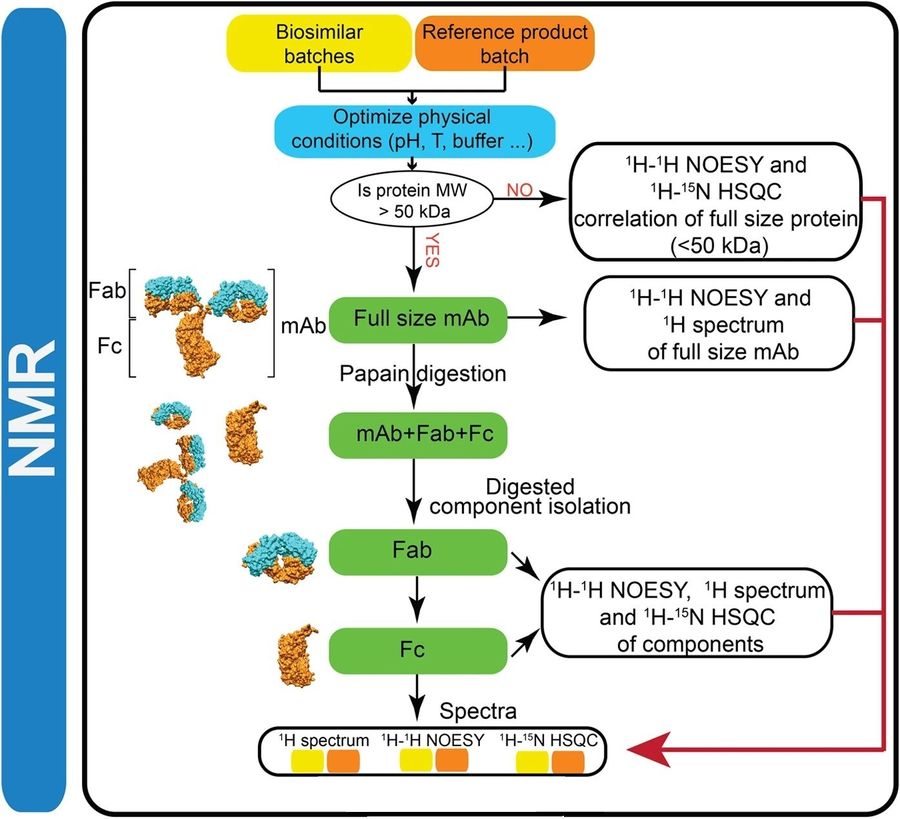 Figure 4. NMR fingerprinting is used for biosimilar structural comparability. (Adapted from Japelj et al., 2016)
Figure 4. NMR fingerprinting is used for biosimilar structural comparability. (Adapted from Japelj et al., 2016)
Formulation and Excipient Compatibility
The formulation of a biopharmaceutical product—comprising buffers, stabilizers, preservatives, and other excipients—can significantly influence its stability and efficacy. NMR spectroscopy is uniquely suited to assess the compatibility between biologic molecules and formulation components. By comparing the NMR spectra of the protein in various formulations, researchers can detect excipient-induced conformational changes or aggregation. For example, 1H NMR can monitor hydrogen bonding interactions, while 2D techniques may uncover subtle structural perturbations. These insights enable the optimization of formulation conditions to enhance shelf-life and minimize adverse interactions.
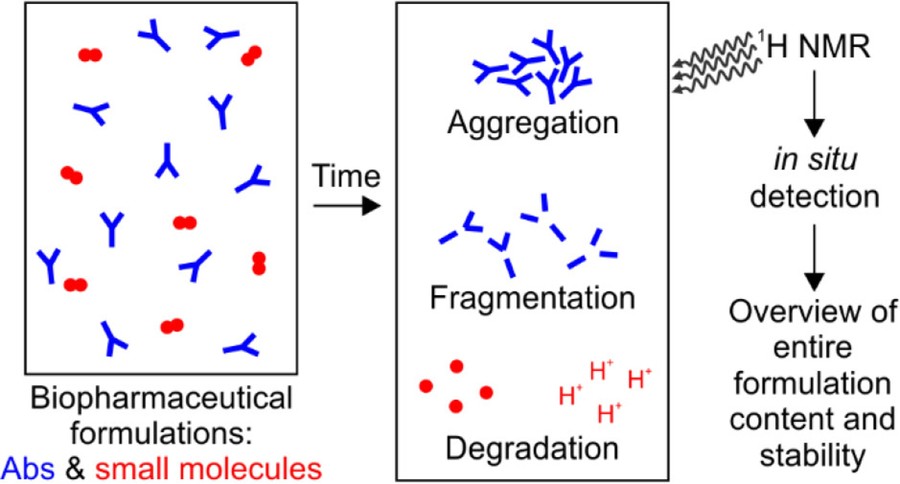 Figure 5. Comprehensive assessment of protein and excipient stability in biopharmaceutical formulations using 1H NMR spectroscopy. (Bramham et al., 2021)
Figure 5. Comprehensive assessment of protein and excipient stability in biopharmaceutical formulations using 1H NMR spectroscopy. (Bramham et al., 2021)
In-Process Control
The application of NMR in upstream process monitoring is gaining traction as bioprocessing becomes more data-driven and quality-focused. Real-time or near-real-time NMR analysis enables the tracking of critical parameters during fermentation or cell culture. Parameters such as nutrient consumption (e.g., glucose, glutamine), byproduct accumulation (e.g., lactate, ammonia), and product expression levels can be observed without interrupting the process. This in-process control provides actionable insights that support timely decision-making, process adjustments, and early detection of deviations. Inline or at-line NMR instruments, when integrated with manufacturing execution systems (MES), support the principles of Process Analytical Technology (PAT) and Quality by Design (QbD).
Host Cell Protein and Metabolite Profiling
Residual host cell proteins (HCPs) and host cell metabolites can impact the safety and efficacy of biopharmaceutical products. NMR-based metabolomics offers a comprehensive, unbiased approach for profiling these contaminants. By generating spectral fingerprints of culture supernatants, researchers can identify metabolite trends that reflect shifts in cell metabolism, stress responses, or contamination events. Additionally, multivariate statistical analysis of NMR data can highlight biomarkers associated with high or low product yield and quality, enabling better control of upstream parameters. As regulatory expectations for impurity profiling grow more stringent, NMR provides a non-targeted yet highly informative method for monitoring host cell-related variables.
Select Service
Related Reading
- Using NMR for Drug Receptor Analysis
- High-Resolution NMR for Complex Molecule Analysis
- NMR Applications in Drug Screening
- Levels of Protein Structure
- Post-Translational Modifications: Types, Functions, and Analytical Methods
- Protein Glycosylation: Classification, Mechanisms, Biological Roles, and Analytical Methods
- What Is Mass Spectrometry
- High-Performance Liquid Chromatography (HPLC)
Advantages and Challenges of NMR Fingerprinting in QC
| Advantages | |
|---|---|
| Non-Destructive | Samples remain intact for further testing. |
| Reproducibility | High inter-laboratory reproducibility under standardized conditions. |
| Structural Insight | Provides both qualitative and quantitative information. |
| Minimal Sample Preparation | Especially for 1D NMR. |
| Versatility | Applicable to proteins, peptides, nucleic acids, and small molecules. |
| Challenges and Limitations | |
| Sensitivity | Generally lower than mass spectrometry; large sample amounts may be required. |
| Instrument Cost and Maintenance | High initial and operational expenses. |
| Expertise Requirement | Skilled operators needed for spectral acquisition and interpretation. |
| Data Complexity | Multidimensional spectra require sophisticated analysis. |
Enhancements and Emerging Trends
Sensitivity Enhancement
One of the longstanding challenges of NMR spectroscopy has been its relatively low sensitivity compared to other analytical techniques such as mass spectrometry (MS). However, recent advancements in hyperpolarization technologies—particularly Dynamic Nuclear Polarization (DNP) and parahydrogen-induced polarization (PHIP)—have brought transformative improvements. DNP, by transferring polarization from unpaired electrons to nuclei, can enhance NMR signal intensities by several orders of magnitude. This breakthrough enables the detection and characterization of low-abundance components such as minor glycoforms, host cell impurities, or degradation products that were previously below the NMR detection threshold. These enhanced sensitivity techniques are also facilitating real-time kinetic studies and early-phase comparability assessments, especially for novel modalities like gene therapies and cell-derived products.
Automation and High-Throughput Analysis
To meet the demands of large-scale biopharmaceutical production, automation and high-throughput capabilities are becoming increasingly integrated into NMR quality control workflows. Modern NMR spectrometers can be equipped with robotic sample changers, temperature control modules, and automated shimming and tuning systems, enabling continuous, unattended operation over extended periods. These instruments, when coupled with sophisticated data acquisition and processing software, allow for the efficient analysis of hundreds of samples per day with minimal manual intervention. Automation not only increases productivity but also reduces human error and enhances reproducibility—critical factors in cGMP-compliant environments. Furthermore, standardized workflows can be developed for routine fingerprinting tasks such as batch comparison, biosimilar equivalence testing, and stability studies.
Integration with Other Techniques
Multimodal analytical platforms are becoming the gold standard in biopharmaceutical characterization, and NMR is increasingly being used in conjunction with complementary techniques to provide a holistic understanding of product quality. For example:
- Mass spectrometry (MS) offers high sensitivity and detailed mass-related structural data, which complements NMR's strength in conformational and dynamic profiling.
- Fourier-transform infrared spectroscopy (FTIR) and Raman spectroscopy provide rapid vibrational fingerprints of biomolecules, useful for secondary structure assessment.
- Chromatographic methods, such as SEC and HIC, contribute valuable information about size heterogeneity and hydrophobicity.
When integrated, these techniques enable multi-dimensional fingerprinting, yielding comprehensive profiles of biopharmaceuticals at both macro- and micro-structural levels. Such synergistic approaches are particularly valuable in comparability studies, forced degradation analysis, and real-time release testing, where a single technique may not capture all quality-relevant attributes.
Chemometric and AI-Driven Analysis
As NMR generates large volumes of high-dimensional data, advanced chemometric and machine learning approaches are increasingly applied to extract actionable insights. Traditional multivariate statistical techniques such as Principal Component Analysis (PCA) and Partial Least Squares Discriminant Analysis (PLS-DA) are used to visualize clustering, detect outliers, and build predictive models for classification and batch differentiation. More recently, artificial intelligence (AI) and deep learning algorithms have been introduced to further enhance the analytical power of NMR fingerprinting.
Select Service
Case Studies
Case 1: Utility of high resolution 2D NMR fingerprinting in assessing viscosity of therapeutic monoclonal antibodies
This study addresses the challenge of high viscosity in concentrated monoclonal antibody (mAb) formulations (≥100 mg/mL), which can complicate stability and subcutaneous delivery during drug development. Recognizing viscosity-prone mAbs early in the development pipeline is crucial. Since higher-order structural features influence viscosity, the study explores noninvasive techniques—Dynamic Light Scattering (DLS) and 2D NMR methyl fingerprinting—as tools to predict and understand viscosity behavior and screen for mitigating excipients.
The researchers analyzed a set of Pfizer mAbs and their Fab and Fc fragments. They found that 2D NMR methyl fingerprinting could distinguish viscosity-prone mAbs from well-behaved ones even at low concentrations (30–40 mg/mL), where bulk viscosity differences are minimal. Viscosity-prone mAbs showed specific NMR features—peak broadening or chemical shift changes—indicative of protein-protein interactions (Fab-Fab or Fab-Fc). These subtle structural changes serve as early indicators of high-viscosity behavior, offering deeper insight beyond traditional viscosity measurements.
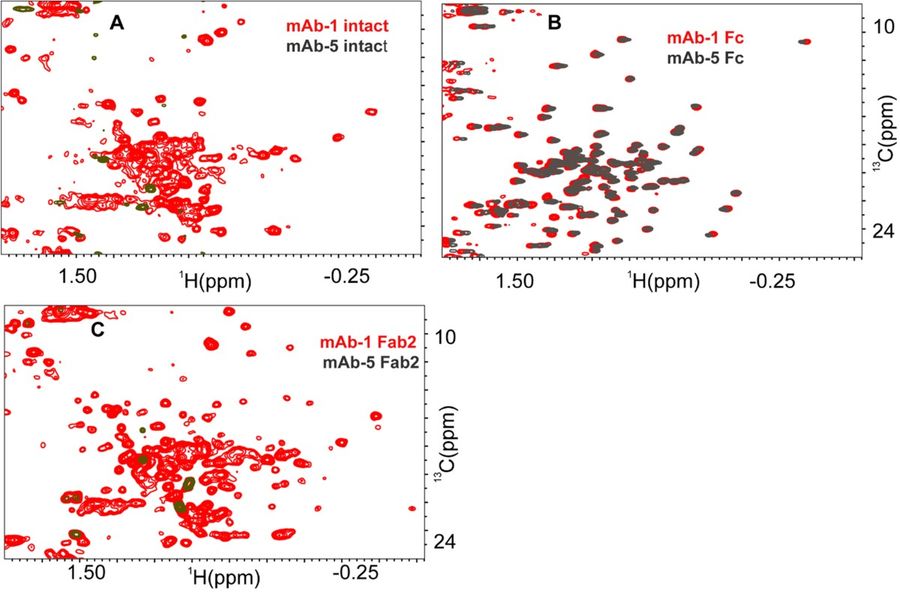 Figure 6. Impact of protein–protein association on HOS fingerprints of intact mAb (A), Fc (B) and Fab2 (C) domain for a well-behaved IgG1 mAb (mAb-1) and viscosity-prone IgG1 mAb (mAb-5). For mAb-5, [1H-13C] HSQC spectra of intact mAb and Fab2 does not yield any cross peaks when compared to those of mAb-1. The [1H-13C] HSQC spectra of Fc fragment for mAb-1 and mAb-5 are similar with respect to number of peaks and peak disposition. (Majumder et al., 2022)
Figure 6. Impact of protein–protein association on HOS fingerprints of intact mAb (A), Fc (B) and Fab2 (C) domain for a well-behaved IgG1 mAb (mAb-1) and viscosity-prone IgG1 mAb (mAb-5). For mAb-5, [1H-13C] HSQC spectra of intact mAb and Fab2 does not yield any cross peaks when compared to those of mAb-1. The [1H-13C] HSQC spectra of Fc fragment for mAb-1 and mAb-5 are similar with respect to number of peaks and peak disposition. (Majumder et al., 2022)
Case 2: NMR spectroscopy for protein higher order structure similarity assessment in formulated drug products
This study highlights the importance of higher order structure (HOS) in peptide and protein drug products (DPs), which is essential for maintaining therapeutic efficacy and safety. For biosimilars or generics, demonstrating structural similarity to the reference product is critical. Solution-state NMR spectroscopy emerges as a powerful, non-invasive method to directly assess HOS in formulated DPs. Despite its potential, few studies have applied NMR to commercial products, and the field lacks standardized, quantitative metrics for comparing structural similarity.
To address this, the researchers analyzed several marketed peptide and protein drugs, including insulin glargine and rituximab, using 1D and 2D NMR techniques. Their results showed clear signatures of structural heterogeneity and oligomerization across products. Quantitative measures such as chemical shift differences, peak intensities, and multivariate analysis (e.g., PCA, Mahalanobis distance) enabled robust comparisons between reference and test DPs. Notably, 2D NMR spectra yielded precise similarity metrics, demonstrating the feasibility of using NMR and chemometric tools to quantitatively assess biosimilarity in real-world formulations.
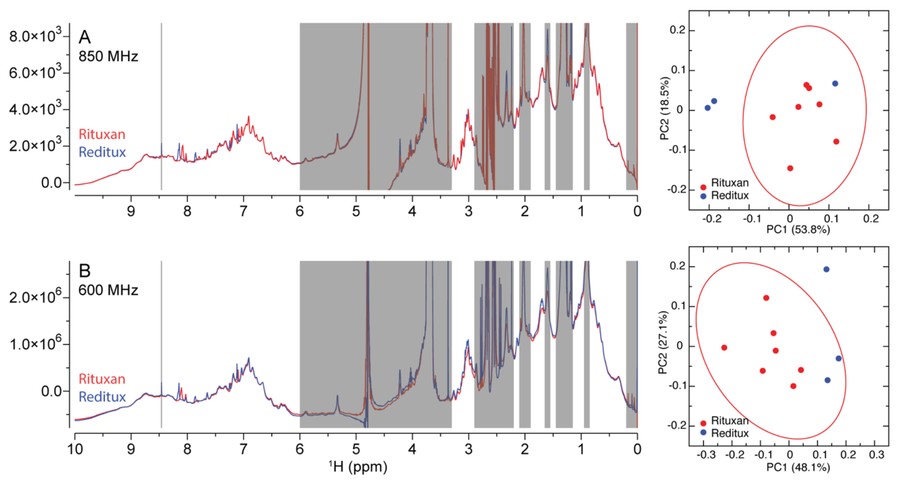 Figure 7. The superimposed 1D 1H NMR spectra of representative rituximab drug products (DP) of Rituxan® and Reditux® collected using 850 MHz (A, left) and 600 MHz (B, left) spectrometers. (Wang et al., 2021)
Figure 7. The superimposed 1D 1H NMR spectra of representative rituximab drug products (DP) of Rituxan® and Reditux® collected using 850 MHz (A, left) and 600 MHz (B, left) spectrometers. (Wang et al., 2021)
In summary, NMR fingerprinting stands as a vital tool in the quality control of biopharmaceuticals. Its ability to provide detailed, reproducible, and non-destructive analysis makes it invaluable for ensuring the safety, efficacy, and consistency of biologic products. While challenges remain, ongoing technological advancements are poised to expand its utility and accessibility. As regulatory expectations evolve and biologics continue to dominate therapeutic pipelines, integrating NMR fingerprinting into standard QC workflows will be critical for future success in the biopharmaceutical sector.
Contact us to learn how our NMR fingerprinting services can support your biopharmaceutical quality control strategy.
References
- Bramham JE, Podmore A, Davies SA, Golovanov AP. Comprehensive assessment of protein and excipient stability in biopharmaceutical formulations using 1H NMR spectroscopy. ACS Pharmacol Transl Sci. 2021;4(1):288-295.
- Japelj B, Ilc G, Marušič J, Senčar J, Kuzman D, Plavec J. Biosimilar structural comparability assessment by NMR: from small proteins to monoclonal antibodies. Sci Rep. 2016;6(1):32201.
- Kang HS, Sattler M. Capturing dynamic conformational shifts in protein-ligand recognition using integrative structural biology in solution. Ubbink M, Perrakis A, eds. Emerging Topics in Life Sciences. 2018;2(1):107-119.
- Maggio RM, Calvo NL, Vignaduzzo SE, Kaufman TS. Pharmaceutical impurities and degradation products: Uses and applications of NMR techniques. Journal of Pharmaceutical and Biomedical Analysis. 2014;101:102-122.
- Majumder S, Bhattacharya DS, Langford A, Ignatius AA.Utility of high resolution 2D NMR fingerprinting in assessing viscosity of therapeutic monoclonal antibodies. Pharm Res. 2022;39(3):529-539.
- Specht T, Arweiler J, Stüber J, Münnemann K, Hasse H, Jirasek F. Automated nuclear magnetic resonance fingerprinting of mixtures. Magnetic Reson in Chemistry. 2024;62(4):286-297.
- Wang D, Zhuo Y, Karfunkle M, et al. NMR spectroscopy for protein higher order structure similarity assessment in formulated drug products. Molecules. 2021;26(14):4251.