Gel Filtration Chromatography
MagHelix™ Protein Purification platform has been established to perform high-quality recombinant protein purification based on our advanced Protein Production Service. Creative Biostructure is not only the world leading protein manufacturer, but also the expert to provide protein purification technical support in order to accelerate your research.
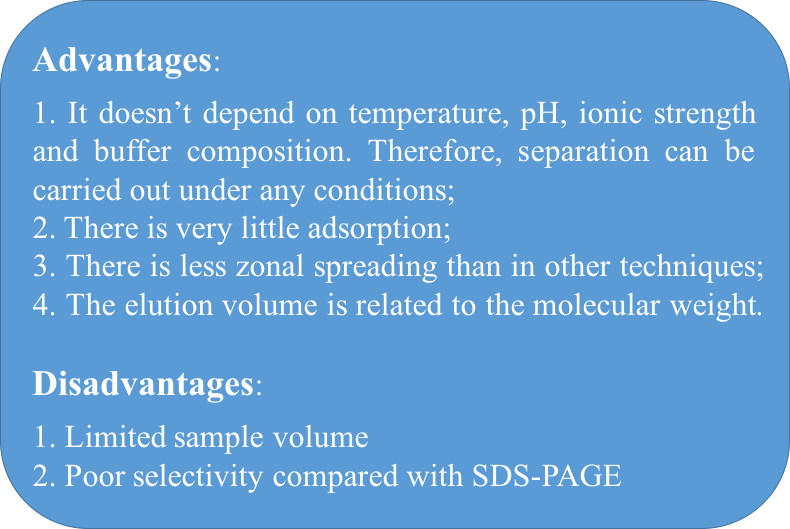
Gel filtration, as known as size exclusion chromatography (SEC), separates proteins according to their different size as they pass through a gel filtration column. Compared to affinity chromatography or ion exchange chromatography, proteins to be seperated by gel filtration chromatography do not need to bind to the chromatography medium, making buffer composition hardly affect resolution. The major advantages and disadvantages of gel filtration chromatography are listed as below:
There are three main applications of gel filtration chromatography:
1. In group separations
Adjusting pH, buffer type, salt concentration during sample preparation;
Removing interfering small molecules (EDTA, Gu-HCl, etc);
Removing small reagent molecules (fluorescent labels, radioactive markers, etc);
But not when the protein will precipitate.
2. In fractionation
Excellent during the polishing stage;
Removes dimers and other aggregates;
Transfers protein to buffer solution ready for the next set of experiments;
Not so suitable if the sample volume is large.
3. For size estimation
Free information;
Gives an estimate of molecular size in any practically any solution;
Precision is not so good.
Troubleshooting:
1. Poor resolution
Reason: Poor packed bed
Recommended solution: Check the efficiency of the column and re-pack if necessary.
Always check the efficiency of a new column, especially one that has been packed in the lab, before using it for critical applications.
Reason: Contamination of the bed with lipids, denatured protein etc.
Recommended solution: Thoroughly clean the column following the recommended protocols given in the instructions provided by the column manufacturer.
2. Poor yields
Reason: Contamination of the bed with lipids, denatured protein etc.
Recommended solution: Thoroughly clean the column following the recommended protocols.
Tip: Running eluent slowly through the column can prevent microbial growth. Cleaning the column thoroughly and keep it under cold conditions in case that the column will not be used for a long time.
3. Elution earlier than expected
Reason: Ionic aggregates and complexes due to carbohydrate chains (glycoproteins).
Recommended solution: Include 150mM NaCl or up to 10% betaine in buffer.
Reason: Complexes associated via SH-groups.
Recommended solution: Add reducing agent (e.g. DTT) to buffer.
Reason: Hydrophobic aggregates.
Recommended solution: Add 10-20% ethanol or other organic solvents to buffer.
Reason: Gel filtration of micelles/protein aggregates.
Recommended solution: Decrease buffer detergent concentration until below CMC or use a different detergent (stay below its CMC).
Remember to check that your sample and your column are stable in the buffer before using special additives to aid solubilisation.
4. Elution later than expected
Reason: Digestion by proteases.
Recommended Solution: Add protease inhibitors to buffer.
Reason: Hydrophobic adsorption.
Recommended Solution: Add 10-20% ethanol or other organic solvents to buffer or decrease salt concentration in high ionic strength buffer.
Reason: Ionic adsorption due to negatively charged groups on the support (Carboxyl-, Sulphate-) and positively charged roups on sample molecules.
Recommended Solution: Include 150mM NaCl (or other salt) in buffer.
TIP: Be sure that your sample is stable in the buffer additive prior to use.
Some additives recommended here may denature or otherwise inactivate a protein.
TIP: Be sure your column and system are stable in the buffer additive prior to use.
Creative Biostructure has a professional support team to provide a wide variety of technical resources in protein purification that are listed as below. We are happy to help you and address your research problems. Please feel free to Contact Us.