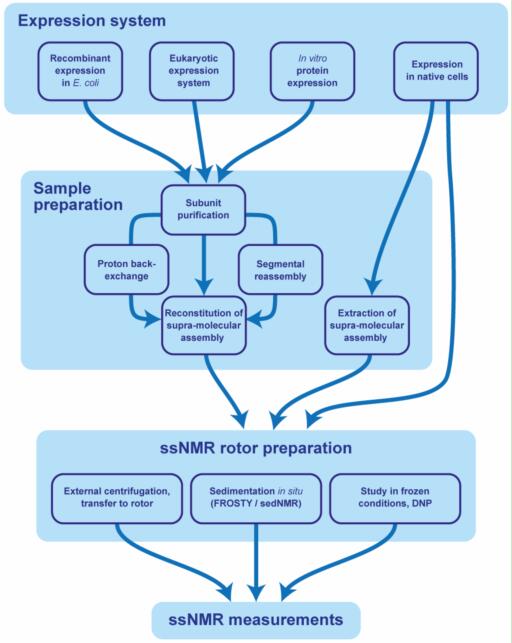
The study of lipid structures within biological membranes is central to our understanding of numerous physiological and pathological processes. Biological membranes, primarily composed of lipid bilayers, serve as dynamic and complex environments essential for cellular function. Nuclear Magnetic Resonance (NMR) spectroscopy has emerged as a vital analytical technique for elucidating the structure, dynamics, and interactions of lipids within these bilayers. Unlike other structural biology tools such as X-ray crystallography or cryo-electron microscopy, NMR permits the investigation of lipids in environments that closely mimic their natural state, offering insights into membrane fluidity, phase behavior, and lipid-protein interactions.
Creative Biostructure offers advanced NMR spectroscopy services for investigating lipid structures in biological membranes, providing in-depth insights into membrane organization, dynamics, and interactions. This article provides a comprehensive overview of the application of NMR spectroscopy in studying lipid structures in biological membranes.
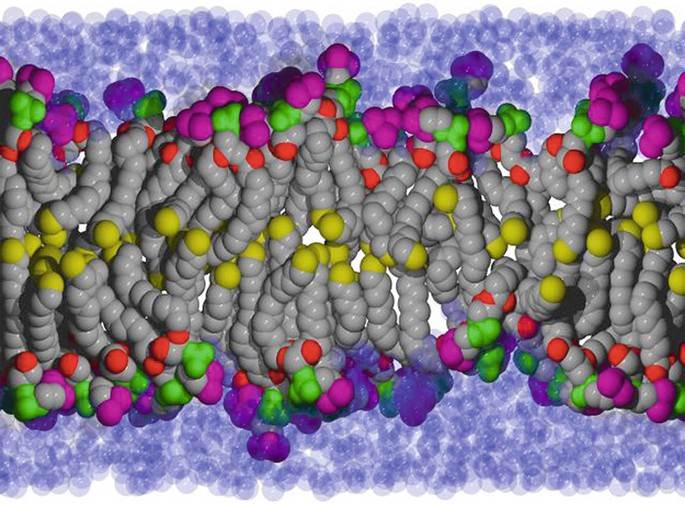
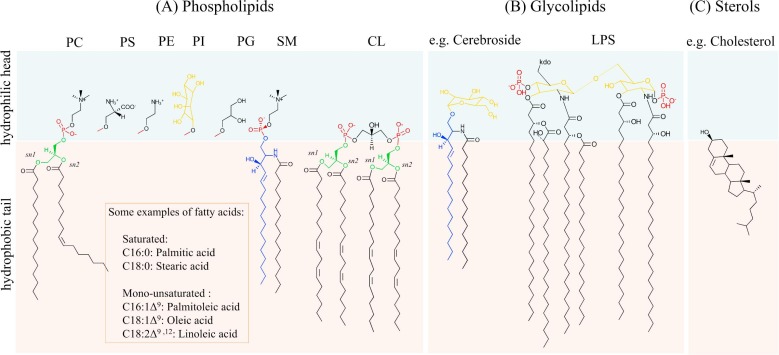
 Figure 1. Using solid-state NMR to study lipid membranes with polyphilic guest (macro)molecules. (Bärenwald et al., 2016)
Figure 1. Using solid-state NMR to study lipid membranes with polyphilic guest (macro)molecules. (Bärenwald et al., 2016)
Basic Principles of NMR Spectroscopy
NMR spectroscopy relies on the magnetic properties of atomic nuclei. Certain nuclei, such as 1H, 13C, 31P, and 15N, possess a property called nuclear spin. When placed in an external magnetic field, these nuclei align according to their spin states. Upon application of a radiofrequency (RF) pulse, the nuclei absorb energy and transition between spin states. The relaxation of these nuclei back to equilibrium results in the emission of RF signals, which are detected and analyzed.
The resonance frequency of a nucleus in an NMR experiment depends on its chemical environment, leading to what is known as the chemical shift. This property enables the identification of distinct molecular components and structural features. Coupled with relaxation times (T1 and T2), scalar couplings (J-couplings), and dipolar couplings, NMR provides a rich dataset for structural analysis.
Relevance of NMR in Lipid Membrane Studies
Lipid bilayers are dynamic systems with various phases and microdomains. The fluidity, heterogeneity, and orientation of lipid molecules influence membrane function. NMR is particularly suitable for lipid studies due to:
- Its ability to probe both static and dynamic aspects of lipid molecules.
- The non-destructive nature of the technique.
- Compatibility with hydrated, intact membrane systems.
- Capability to resolve individual lipid components and monitor molecular motion.
These features make NMR ideal for characterizing lipid order, chain packing, headgroup orientation, and interactions with proteins or other small molecules.
Sample Preparation for NMR Studies of Lipids
Preparing biologically relevant lipid samples is critical for meaningful NMR analysis. Common lipid sample formats include:
- Small Unilamellar Vesicles (SUVs): Useful for solution NMR but often less representative of natural membrane systems.
- Large Unilamellar Vesicles (LUVs): Closer in structure to natural membranes; amenable to both solution and solid-state NMR.
- Multilamellar Vesicles (MLVs): Provide a stack of bilayers, ideal for 31P and 2H solid-state NMR.
- Oriented Membrane Samples: Lipid bilayers aligned on glass plates or stretched gels for studying lipid orientation relative to the bilayer normal.
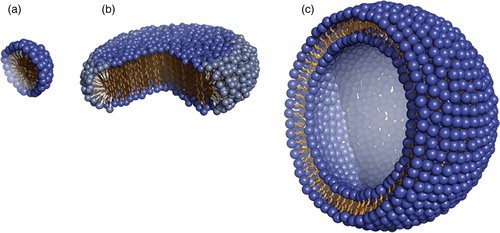
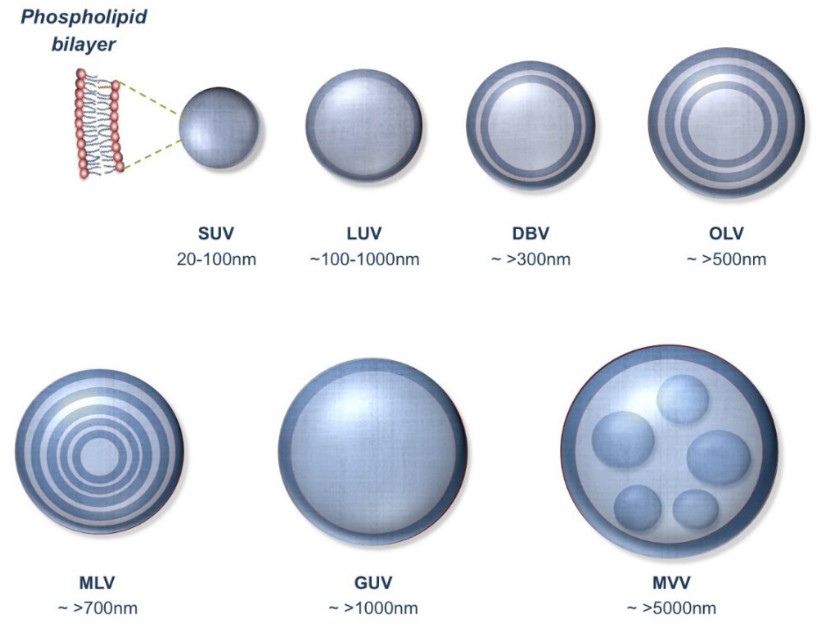 Figure 2. Different types of lipid vesicles. SUV: small unilamellar vesicle; LUV: large unilamellar vesicle; DBV: double bilayer vesicle; OLV: oligolamellar vesicle; MLV: multilamellar vesicle; GUV: giant unilamellar vesicle; MVV: multivesicular vesicle. (Mozafari et al., 2021)
Figure 2. Different types of lipid vesicles. SUV: small unilamellar vesicle; LUV: large unilamellar vesicle; DBV: double bilayer vesicle; OLV: oligolamellar vesicle; MLV: multilamellar vesicle; GUV: giant unilamellar vesicle; MVV: multivesicular vesicle. (Mozafari et al., 2021)
Sample purity, lipid composition, hydration level, and isotopic labeling (e.g., 13C, 2H, 15N) must be carefully controlled.
Structural Information Derived from NMR
NMR yields a multitude of structural parameters:
- Chemical Shifts: Reflect the electronic environment of nuclei, used for headgroup identification and chain conformation.
- Order Parameters (S): Derived from 2H NMR, these indicate the degree of order within lipid acyl chains.
- Quadrupolar Splitting: Provide information on headgroup orientation and membrane fluidity.
- Dipolar Couplings: Reflect distances and angles between nuclear spins, useful for determining spatial relationships.
- Relaxation Times (T1, T2): Indicate molecular mobility.
NMR-Active Nuclei in Lipid Studies
Several NMR-active nuclei are employed in membrane lipid research:
- 1H NMR: Offers high sensitivity and is widely used for detecting proton environments in lipid molecules. However, spectral overlap can be an issue.
- 13C NMR: Provides detailed information on carbon backbones of lipids. Often used in combination with isotopic labeling.
- 31P NMR: Extremely useful for probing phospholipid headgroups, offering insights into headgroup orientation and membrane phase behavior.
- 2H NMR: Utilized with deuterated lipids to study lipid acyl chain dynamics and order parameters.
Types of NMR Techniques for Lipid Membranes
Several NMR modalities are used to interrogate membrane-associated lipids, each offering unique advantages:
| Technique | Description |
|---|---|
| Solution-State NMR | Solution-state NMR is typically limited to studies of small unilamellar vesicles (SUVs) or lipid micelles, where fast molecular tumbling permits high-resolution spectra. It is suitable for:
|
| Solid-State NMR | Solid-state NMR is crucial for studying larger lipid assemblies such as multilamellar vesicles (MLVs) and oriented lipid bilayers:
|
| Magic Angle Spinning (MAS) | MAS is used to average out anisotropic interactions in solid samples by spinning them at an angle of 54.74° (the magic angle) relative to the magnetic field. MAS enhances spectral resolution and is valuable for:
|
| Dynamic Nuclear Polarization (DNP) | DNP enhances NMR signal sensitivity by transferring polarization from unpaired electron spins to nuclei. This method is gaining popularity for lipid studies where sensitivity is a limiting factor. |
Select Service
- NMR Spectroscopy Services
- X-ray Crystallography Services
- Combinational Applications of Cryo-EM, X-ray Crystallography and NMR
- Cryo-Electron Microscopy (Cryo-EM) Services
- Characterization of Peptide Structures and Peptide-Lipid Interactions in Membranes
- Characterization of Lipid Membranes
- Solution-state NMR Service
- Solid-state NMR Services
- Membrane Protein Structure Determination by Solid-state NMR
- Magic-Angle Spinning (MAS) Solid-state NMR Service
Applications of NMR in Lipid Membrane Research
Membrane Phase Behavior
NMR provides critical insight into lipid phase transitions, which are essential for understanding membrane thermodynamics and function. 31P NMR, due to its sensitivity to the orientation and motion of phosphate groups, reveals distinct spectral line shapes corresponding to different phases:
- Lamellar phase (Lα): Typically exhibits a broad asymmetric powder pattern.
- Hexagonal phase (HII): Produces a characteristic inverted cone-shaped spectrum.
- Isotropic phase: Appears as a narrow, symmetric line due to rapid motion and lack of orientational order.
2H NMR can further delineate phase boundaries by showing changes in order parameters across temperature or composition gradients. This approach is widely used to study phase transitions in synthetic and natural lipid systems, including domain formation in complex mixtures.
Lipid Rafts and Microdomains
Biological membranes are not homogenous; they contain microdomains, often referred to as lipid rafts, enriched in cholesterol and sphingolipids. These domains play pivotal roles in signaling, trafficking, and pathogen entry.
NMR techniques such as MAS and 2H NMR provide direct evidence for domain heterogeneity:
- Lipid molecules in cholesterol-rich regions exhibit higher order parameters, indicative of tighter packing.
- Differences in relaxation behavior and spectral resolution help distinguish raft from non-raft domains.
- By using isotopically labeled lipids or cholesterol, researchers can track domain-specific dynamics and interactions.
Moreover, NMR facilitates the quantification of domain sizes, lifetimes, and their responses to changes in temperature, ionic strength, or protein insertion.
Protein-Lipid Interactions
Integral and peripheral membrane proteins influence lipid organization and, conversely, are modulated by the lipid environment. NMR offers multiple strategies for elucidating these interactions:
- 31P and 2H NMR: Reveal perturbations in lipid headgroup orientation and acyl chain order upon protein binding.
- Chemical shift perturbations (CSPs): Monitored in 1H or 13C spectra, CSPs indicate direct contact sites and conformational rearrangements.
- Saturation Transfer Difference (STD) NMR: Identifies binding epitopes by detecting magnetization transfer from proteins to nearby lipids.
- Paramagnetic Relaxation Enhancement (PRE): Applied to detect proximity between protein residues and specific lipid atoms.
These approaches yield information on binding stoichiometry, affinity, insertion depth, and even structural remodeling induced by protein association.
Drug-Membrane Interactions
The membrane-associative properties of pharmaceutical compounds strongly influence their bioavailability and mechanism of action. NMR methods allow the real-time study of drug partitioning, localization, and effects on membrane integrity:
- 1H and 13C MAS NMR: Provide detailed profiles of drug distribution within bilayers.
- 31P NMR: Detects changes in headgroup mobility and orientation upon drug incorporation.
- Relaxation studies: Assess the influence of drugs on lipid dynamics and membrane viscosity.
- NOESY and ROESY experiments: Identify close contacts between drug protons and lipid segments.
Such insights are critical for optimizing drug delivery systems, designing lipid-based carriers (e.g., liposomes), and predicting membrane permeability and toxicity profiles.
Lipid Dynamics and Diffusion
Membrane fluidity and lipid mobility are crucial for cellular processes such as vesicle trafficking, fusion, and signaling. NMR elucidates these dynamic properties through:
- Spin-lattice (T1) and spin-spin (T2) relaxation times: Quantify segmental motions and phase transitions.
- Exchange spectroscopy (EXSY): Tracks lateral diffusion of lipids between domains or across bilayers.
- Stimulated Echo (STE) and Pulsed Gradient Spin Echo (PGSE) experiments: Measure diffusion coefficients of lipids and small molecules.
- Flip-flop rates: Determined by monitoring the movement of lipids between bilayer leaflets, often using 31P or 2H labeling.
Together, these experiments provide a temporal and spatial map of membrane dynamics, informing on how lipid composition and external factors (e.g., temperature, pH, ions) influence bilayer behavior.
Select Service
- Characterization of Peptide Structures and Peptide-Lipid Interactions in Membranes
- Characterization of Lipid Membranes
- Interactions between Membrane Lipids and Membrane Proteins
- Membrane Protein Structure Determination by Solid-state NMR
- Solution-state NMR Service
- Solid-state NMR Services
- Magic-Angle Spinning (MAS) Solid-state NMR Service
- Saturation Transfer Difference (STD) NMR Service
Case Studies
Case 1: NMR insights into basil essential oil's disruption of Listeria cell membranes
Basil essential oil (BEO) shows strong antibacterial activity against Listeria monocytogenes, eliminating detectable bacteria within 8 hours at 1 mg/mL. Spectroscopic and microscopy analyses revealed that BEO disrupts the phospholipid bilayer, increasing membrane permeability and causing leakage of proteins and DNA. It also inhibits key enzymes in the EMP glycolytic pathway, likely via hydrophobic interactions between α-bergamotene and phosphofructokinase or pyruvate kinase. Additionally, BEO induces oxidative stress through ROS generation.
To confirm membrane interactions, 1H NMR spectroscopy was used to verify that linalool, a BEO component, targets lipid components of the bacterial membrane. As NMR is a powerful tool for studying membrane structure and drug interactions, this confirms its value in elucidating natural antimicrobial mechanisms.
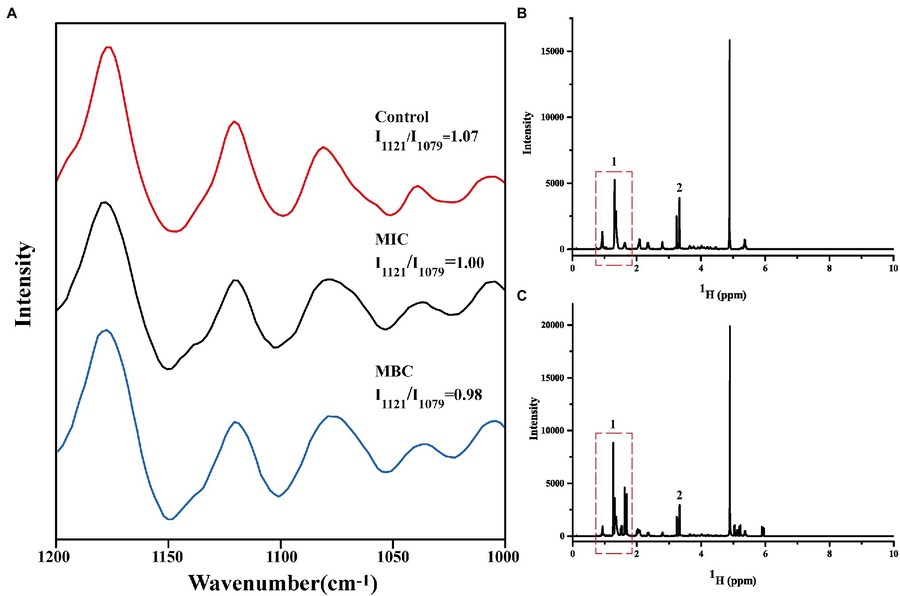 Figure 3. Changes in Raman spectra of cell membrane phospholipids (A); One-dimensional 1H NMR spectra of cell membrane phospholipids before (B) and after (C) reaction with linalool. (Li et al., 2022)
Figure 3. Changes in Raman spectra of cell membrane phospholipids (A); One-dimensional 1H NMR spectra of cell membrane phospholipids before (B) and after (C) reaction with linalool. (Li et al., 2022)
Case 2: NMR and MD reveal cholesterol organization in bilayers
This study uses solid-state NMR spectroscopy alongside molecular dynamics (MD) simulations to uncover the molecular structure of cholesterol clusters within phospholipid bilayers—an area previously lacking direct structural insight. Using 2D 13C–13C correlation spectroscopy with differentially labeled cholesterol, researchers discovered that cholesterol self-associates in a face-to-face orientation, forming nanoscale oligomers at concentrations ranging from 17% to 44% mol.
Spin-counting experiments using 13C and 19F NMR enabled precise quantification of cluster sizes: at ~20% cholesterol, dimers dominate, while at 44% (typical of viral envelopes and liquid-ordered membrane domains), both dimers and tetramers are present. These dimers were found in both sphingomyelin-rich and -poor membrane systems, suggesting a universal behavior.
MD simulations corroborated the NMR findings, revealing the lifetimes, stability, and structural organization of these cholesterol clusters. The data collectively support that dimers are the fundamental structural unit of cholesterol in membranes, providing a new structural framework for understanding cholesterol's interaction with membrane proteins and its role in disease pathogenesis.
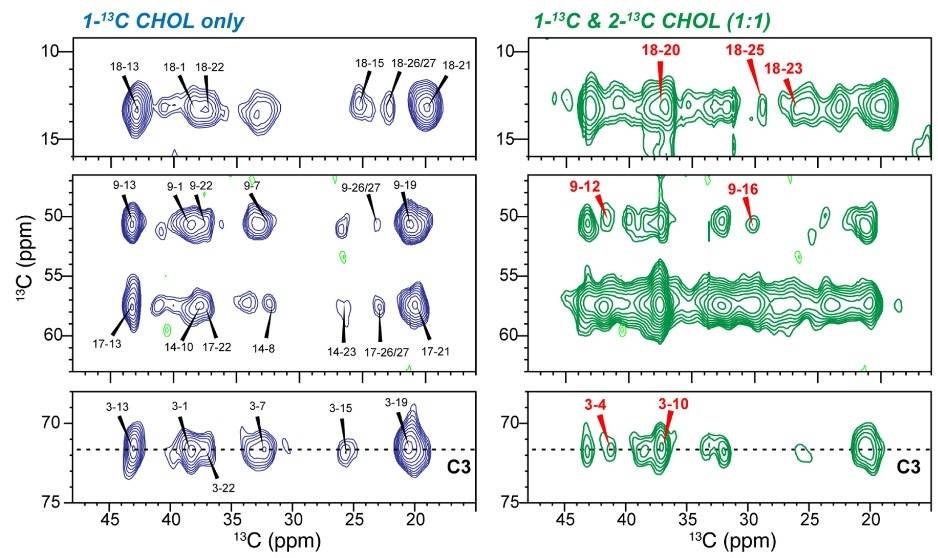 Figure 4. Two-dimensional 13C-13C correlation spectra revealing cholesterol self-association in lipid membranes. 2D spectra of the 44% CHOL VM+ membrane, measured at 240 K using a 13C spin diffusion mixing time of 750 ms. (Elkins et al., 2021)
Figure 4. Two-dimensional 13C-13C correlation spectra revealing cholesterol self-association in lipid membranes. 2D spectra of the 44% CHOL VM+ membrane, measured at 240 K using a 13C spin diffusion mixing time of 750 ms. (Elkins et al., 2021)
Advantages and Limitations
Advantages:
- Non-invasive and non-destructive.
- Provides both structural and dynamic information.
- Applicable to intact and hydrated membrane systems.
- Can differentiate among lipid types and phases.
Limitations:
- Requires high sample concentrations.
- Spectral overlap in complex mixtures.
- Time-consuming data acquisition and analysis.
- Sensitivity is lower compared to other techniques without enhancement methods like DNP.
In summary, NMR spectroscopy stands as a cornerstone technique in the study of lipid structures within biological membranes. By leveraging a suite of nuclei and experimental modalities, NMR affords unparalleled insights into lipid organization, phase behavior, and interaction dynamics. Despite certain limitations, continual methodological enhancements are broadening the scope of NMR in membrane biophysics. As the complexity of biological membrane models increases to more closely resemble native systems, NMR will undoubtedly remain pivotal in decoding the structural subtleties of lipid assemblies and their roles in cellular physiology and pathology.
Ready to decode the complexities of lipid structures with precision and clarity? Partner with us at Creative Biostructure, where advanced NMR technology meets expert analysis. Our comprehensive NMR services are tailored to unravel the intricate organization and dynamics of biological membranes, supporting your research in membrane biophysics, cell signaling, drug-membrane interactions, and beyond. Contact us today and let NMR illuminate the structural landscape of your lipid research.
References
- Bärenwald R, Achilles A, Lange F, Ferreira T, Saalwächter K. Applications of solid-state NMR spectroscopy for the study of lipid membranes with polyphilic guest (Macro)molecules. Polymers. 2016;8(12):439.
- Elkins MR, Bandara A, Pantelopulos GA, Straub JE, Hong M. Direct observation of cholesterol dimers and tetramers in lipid bilayers. J Phys Chem B. 2021;125(7):1825-1837.
- Li C, Zhang C, Chen X, Cui H, Lin L. The interference mechanism of basil essential oil on the cell membrane barrier and respiratory metabolism of listeria monocytogenes. Front Microbiol. 2022;13.
- Mozafari MR, Mazaheri E, Dormiani K. Simple equations pertaining to the particle number and surface area of metallic, polymeric, lipidic and vesicular nanocarriers. Sci Pharm. 2021;89(2):15.