MemPro™ Sulfatases Services
Creative Biostructure provides custom gene-to-structure services for sulfatases.
Sulfatases, alternative name arylsulfatases C (ASC) which cleave sulfate esters in biological systems, play a key role in sulfate regulation, hormone regulation, cellular degradation and modulation of signaling pathways. Sulfatase substrates range from small cytosolic steroids, such as estrogen sulfate, to complex cell-surface carbohydrates, such as the glycosaminoglycans. Sulfatases have a highly conversed protein sequences and structures. Their N-terminal regions contain the consensus sulfatase motifs and a unique active-site aldehyde residue, formylglycine (FGly), which is installed post-translationally. Sulfatases show a “mushroom-like shape” which refers to two antiparallel alpha-helices protrude from the roughly spherical molecule.
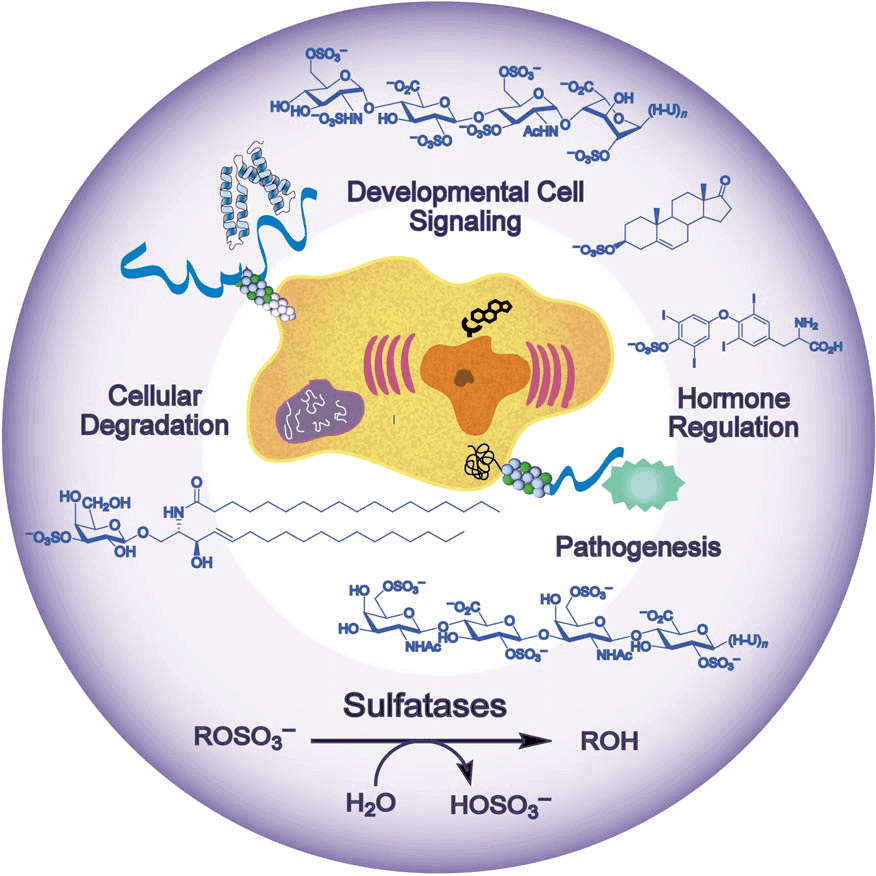 Figure 1. sulfatases biological process
Figure 1. sulfatases biological process
Estrone sulfatase (ES) is responsible for maintaining high levels of estrogens during pregnancy and also in breast tumor cells. ES is found in the microsomal fraction of human placenta. The membrane-bound enzyme is distributed in the rough endoplasmic reticulum (ER) including the perinuclear cisternae, the Golgi cisternae, the endocytic structures, and the plasma membrane. ES catalyzes the hydrolysis of estrone sulfate, which is subsequently reduced to 17β-estradiol by 17HSD1. They account for most of the estrogens in postmenopausal women and, hence, could be a major factor in hormonal breast cancers. Selective estrogen enzyme modulators that inhibit these enzymes have shown promise as anti-tumor agents in hormonal breast carcinoma.
Heparan sulfate (HS) is an important mediator in a lot of biological process including embryogenesis and developmental signaling, inflammation, coagulation, angiogenesis, cancer metastasis, and microbial and viral adherence and invasion. HS has a very complex oligosaccharides sequences which qualifies itself as one of the most information-dense biomolecules. Sulfatase enzymes are invaluable techniques to decode HS sequences since HS contains quite distinct disaccharide units owing to variations in glycosidic connection, epimerization, and sulfonation.
As is the case for many processes in carbohydrate chemistry, the sulfonation of saccharides in a regioselective manner often requires arduous protecting-group manipulations. Sulfatases might be a viable alternative for regioselective sulfonation. They catalyze the synthesize of sulfation compounds and subsequently convert them into glycosyl donors for the construction of more complex sulfated oligosaccharides.
Additionally, FGly is essential for catalytic activity. When post-translation modification of FGly is deficient, multiple sulfatase deficiency (MSD) will be speculated including X-linked ichthyosis (XLI), chondrodysplasia (CDPX), leukodystrophy (MLD) and etc.
References:
Hanson S R, Best M D, Wong C H. Sulfatases: structure, mechanism, biological activity, inhibition, and synthetic utility[J]. Angewandte Chemie International Edition, 2004, 43(43): 5736-5763.
Hernandez-Guzman F G, Higashiyama T, Pangborn W, et al. Structure of human estrone sulfatase suggests functional roles of membrane association[J]. Journal of Biological Chemistry, 2003, 278(25): 22989-22997.
