Structural Research of Integrin Adhesion Receptors
Integrin adhesion receptors are vital cell surface proteins that play a fundamental role in mediating cellular adhesion, signal transduction, and cell migration. These receptors are heterodimeric complexes composed of α and β subunits, with one of the most prominent representatives being integrin αIIbβ3.
Integrin αIIbβ3, primarily expressed on platelets, is an indispensable receptor for blood clotting and hemostasis. Despite considerable research efforts, inhibiting the physiological activation pathways of this receptor does not always prevent fatal thrombosis, indicating the existence of additional, unidentified pathways. Researchers have delved into the structural basis of this phenomenon and recently made a groundbreaking discovery. They identified a unique structural motif that safeguards integrin αIIbβ3 by selectively destabilizing its inactive state.
At the extracellular membrane border, an overpacked αIIb(W968)-β3(I693) contact disrupts the optimal assembly of the αIIbβ3 transmembrane complex, thereby maintaining the receptor in its inactive state. This destabilization of approximately 1.0 kcal/mol can be mitigated by hydrodynamic forces but not by physiological agonists. Thus, hydrodynamic forces are identified as a pathological activation stimulus for integrin αIIbβ3. The triggering of this safeguard solely by pathological stimuli effectively increases the free energy barrier between inactive and active receptor states, without elevating the risk of bleeding. These findings shed light on the evolutionary adaptations of integrin αIIbβ3 to protect the healthy cardiovascular system from fatal, hydrodynamic force-mediated activation.
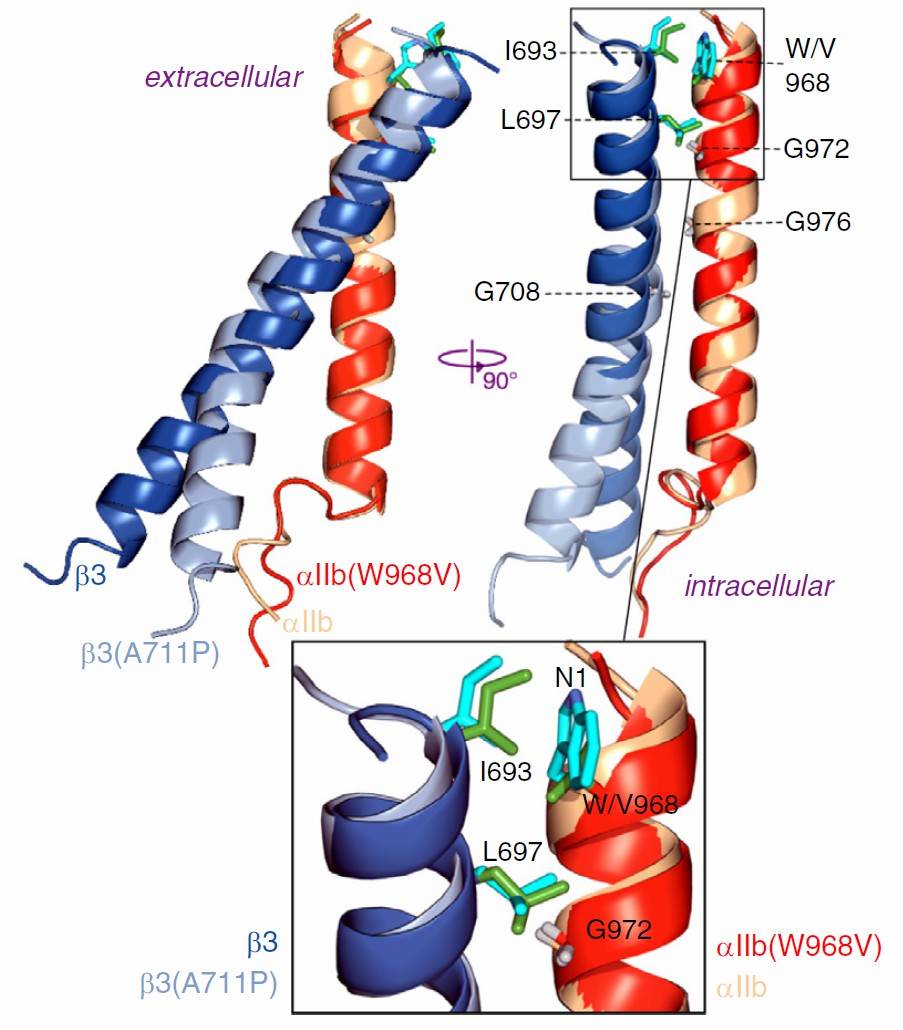 Figure 1. Structure of the integrin αIIb(W968V) β3 TM complex. (Situ A J, et al., 2021)
Figure 1. Structure of the integrin αIIb(W968V) β3 TM complex. (Situ A J, et al., 2021)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Human Integrin αIIbβ3 transmembrane-cytoplasmic heterodimer (expressed in Escherichia coli) | Homo sapiens | Solution NMR | / | 2KNC |
| Integrin αIIb(W968V) β3 transmembrane complex (expressed in Escherichia coli) | Homo sapiens | Solution NMR | / | 7KN0 |
Table 1. Structural research of integrin adhesion receptors.
Creative Biostructure offers a wide range of cutting-edge structural analysis services to investigate the intricacies of integrin adhesion receptors. Through state-of-the-art technologies such as X-ray crystallography, cryo-electron microscopy (cryo-EM), and nuclear magnetic resonance (NMR) spectroscopy, our expert team can determine high-resolution structures of these receptors. The utilization of these advanced techniques empowers researchers to visualize the molecular details of integrin αIIbβ3, gaining valuable insights into its conformational changes upon activation.
Furthermore, the structural analysis services at Creative Biostructure encompass not only the determination of static structures but also dynamic studies. Molecular dynamics simulations and computational modeling are employed to examine the receptor's behavior in response to mechanical forces and agonist binding. Contact us to explore the ways in which our capabilities can empower your research endeavors and bring you closer to accomplishing your scientific objectives.
References
- Yang J, et al. Structure of an integrin αIIbβ3 transmembrane-cytoplasmic heterocomplex provides insight into integrin activation. Proceedings of the National Academy of Sciences. 2009, 106(42): 17729-17734.
- Situ A J, et al. Insight into pathological integrin αIIbβ3 activation from safeguarding the inactive state. Journal of Molecular Biology. 2021, 433(7): 166832.