Structural Research of Caliciviridae
The Caliciviridae is a group of small, enveloped viruses with 7.4-8.3 kb linear positive-sense RNA genomes that are major pathogens of gastroenteritis and respiratory infections in humans and animals, which is of increasing concern in immunocompromised individuals. The Caliciviridae contains four recognised genera, Norovirus, Sapovirus, Lagovirus, and Vesivirus. Of these, the most clinically essential representative is human norovirus, which is the main cause of acute gastroenteritis. In recent years, sequence analysis and structural biology analyses have determined the three-dimensional structures of a range of caliciviruses, which have been used to understand their replication strategies, thus providing a basis for the design of novel antiviral compounds.
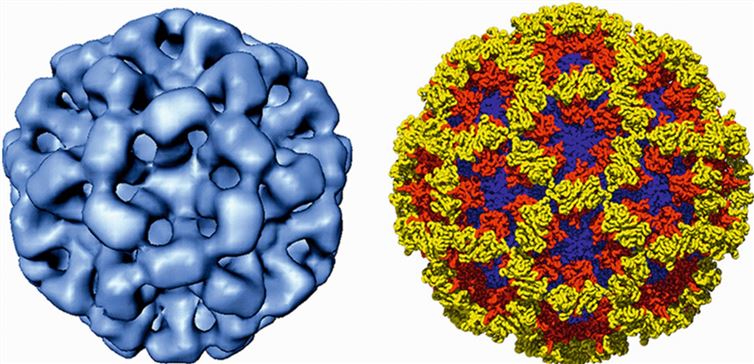 Figure 1. Cryo-image (left) of the Norwalk virus-like particle and X-ray structure (right) of its capsid. (Vinjé J, et al., 2019)
Figure 1. Cryo-image (left) of the Norwalk virus-like particle and X-ray structure (right) of its capsid. (Vinjé J, et al., 2019)
Genome Structure of the Calicivirus
The calicivirus genome consists of 7.4-8.3 kb of linear positive ssRNA molecules containing 2-4 open reading frames, ORF1, ORF2, ORF3, and ORF4. The viral proteogenome linkage (VPg) is covalently bound to the 5'-terminal and 3'-poly(A)-tails. The 5'-proximal ORF1 encodes a large polyprotein, which is subsequently co-translated and cleaved by the protease NS6 pro into at least six non-structural proteins, NS1/2, NS3, NS4, NS5, NS6, and NS7. The 2.2-2.4 kb subgenomic RNA (sgRNA) is synthesized intracellularly and ligated by VPg.
Characteristic Viral Particle Morphology of Caliciviruses
In recent years, researchers have used cryo-electron microscopy (cryo-EM), X-ray crystallography, and 3D image reconstruction to resolve the structure of caliciviruses at sub-nanometre resolution. The structure shows that the virus particles are unenveloped, with a diameter of about 35-40 nm and icosahedral symmetry. The capsid consists of 90 dimers of the major structural protein VP1 arranged on a T=3 icosahedral lattice. The VP1 is divided into two structural domains, S and P. The S domain constitutes the continuous bottom of the capsid and has a β-barrel motif. The P structural domain protrudes from the surface of the capsid to form an arch, which is further divided into the P1 and P2 sub-structural domains. P2 is an insertion fragment in P1, located in the outermost layer of the capsid, and is the site of several neutralization epitopes.
Virus-like particles (VLP) have developed into a widely accepted technology, particularly in the field of vaccinology. Creative Biostructure as a leading biological company offers a comprehensive range of virus-like particles (VLPs) products and virus-like particle (VLP) services for a wide range of applications. Our Caliciviridae virus-like particle products help researchers advance caliciviruses virology-related research, including basic research on viral infections, diagnostics, vaccines, and antiviral drug development.
| Cat No. | Product Name | Virus Name | Source | Composition |
| CBS-V106 | Group 2 norovirus VLP (Core protein) | Norovirus | Mammalian cell recombinant | Core protein |
| CBS-V702 | Norovirus GI.1 VLP (VP1 Protein) | Norovirus | Insect cell recombinant | VP1 |
| CBS-V707 | Norwalk virus VLP (CP Protein) | Norwalk virus | Yeast recombinant | Capsid protein |
| Explore All Caliciviridae Virus-like Particle Products | ||||
Creative Biostructure is a leading structural analysis company for biomacromolecules. We have cutting-edge technology for virus-like particle construction as well as characterize the structural integrity and stability of newly synthesized VLPs. Our scientists have expertise in structural characterization and are skilled in viral structure identification techniques such as cryo-em for viral particle identification and characterization, helping clients accelerate their research progress efficiently and cost-effectively.
In addition, we offer a range of virus-like particles (VLPs) products, which are self-assembling structures that mimic the morphology and surface characteristics of viruses, making them ideal tools for research on virus entry, immunology, and vaccine development. Please do not hesitate to contact us if you are interested in our service and products. Our team is committed to providing high-quality, reliable, and customizable VLP products to support your scientific endeavors.
References
- Vinjé J, et al. ICTV Virus Taxonomy Profile: Caliciviridae. J Gen Virol. 2019. 100(11): 1469-1470.
- Lee JH, et al. Structure and Function of Caliciviral RNA Polymerases. Viruses. 2017. 9(11): 329.
- Chen R, et al. X-ray structure of a native calicivirus: structural insights into antigenic diversity and host specificity. Proc Natl Acad Sci U S A. 2006. 103(21): 8048-8053.