Structural Research of HMG CoA Reductase
Cholesterol, an essential lipid in mammals, serves as a structural component of cell membranes and is a precursor for numerous vital molecules. The rate-limiting enzyme in cholesterol biosynthesis is 3-Hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), a key target for cholesterol-lowering statin drugs. Understanding the structural intricacies of HMGCR and its interactions is critical for developing novel therapeutic strategies to combat dyslipidemia and related cardiovascular diseases.
Recent research has significantly advanced our knowledge of the structure of HMG CoA reductase, with particular attention to its interaction with UBIAD1. This interaction plays a pivotal role in the regulation of cholesterol synthesis. Cryo-electron microscopy (Cryo-EM) has emerged as a powerful technique in unraveling the HMGCR-UBIAD1 complex. Utilizing this technique, researchers have made groundbreaking strides in elucidating the structural dynamics of this complex.
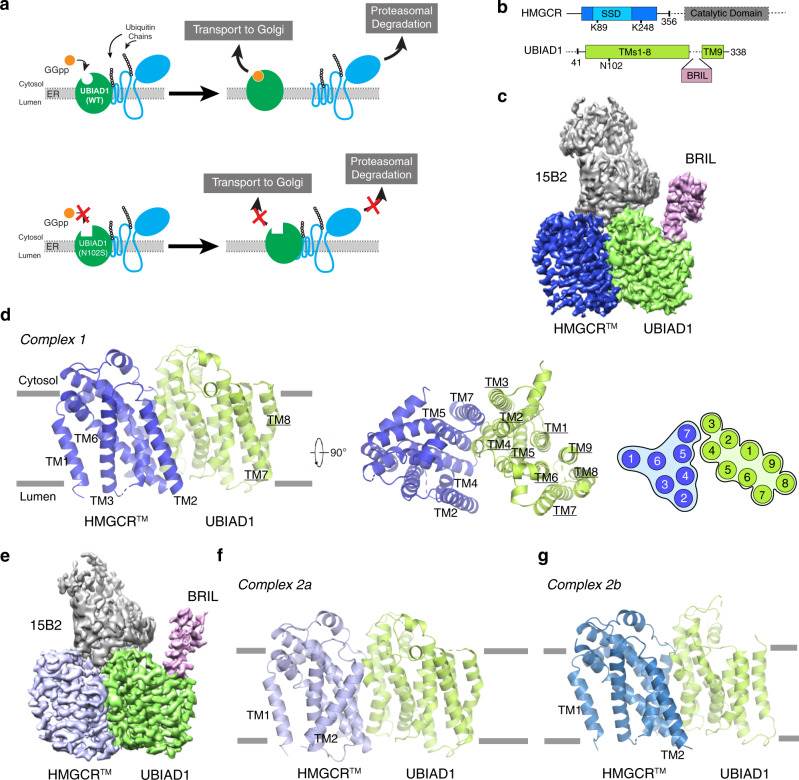 Figure 1. Cryo-EM structure of the HMGCRTM-UBIAD1N102S complex. (Chen H, et al., 2022)
Figure 1. Cryo-EM structure of the HMGCRTM-UBIAD1N102S complex. (Chen H, et al., 2022)
Cryo-Electron Microscopy Unveils the HMGCR-UBIAD1 Complex
Researchers employed cryo-electron microscopy to capture high-resolution structures of the HMGCR-UBIAD1 complex. The intricate network of interactions between HMGCR and UBIAD1 has been visualized, shedding light on the mechanism underlying cholesterol synthesis regulation. The cryo-EM structures revealed that the complex is maintained through interactions between specific transmembrane helices (TM) of HMGCR and UBIAD1. Notably, disrupting this interface by mutagenesis hinders complex formation, leading to an increase in HMGCR degradation. This finding emphasizes the significance of the HMGCR-UBIAD1 interaction in fine-tuning cholesterol homeostasis.
The Role of Sterol Sensing Domain (SSD) in HMGCR Regulation
Further investigations into the HMGCR structure have unraveled a crucial element within the enzyme - the Sterol Sensing Domain (SSD). Comprising TMs 2-6 of HMGCR, this 170-amino acid region exhibits two distinct conformational states. One of these conformations is pivotal in initiating HMGCR degradation. The SSD acts as a sensor for cellular sterol levels. When cellular sterol concentration is high, HMGCR undergoes a conformational change, enabling recruitment of specific proteins that facilitate its degradation. This process represents a key reaction in the regulatory system that governs cholesterol synthesis.
| Protein | Organism | Method | Resolution | PDB Entry ID |
| HMGCR-UBIAD1 Complex State 1 (expressed in HEK293 cells) | Cricetulus griseus, Escherichia coli, Mus musculus | Cryo-EM single particle analysis | 3.23 Å | 8DJM |
Table 1. Structural research of HMG CoA reductase.
As pioneers in the field of structural biology, Creative Biostructure offers cutting-edge structural analysis services. Our expertise in cryo-electron microscopy (cryo-EM) allows us to determine the high-resolution structures of protein complexes, such as the HMGCR-UBIAD1 complex. Leveraging our state-of-the-art technologies and in-depth understanding of structural biology, we provide comprehensive insights into the molecular mechanisms underpinning various biological processes. Contact us to discover how our cutting-edge capabilities can empower your research and drive you closer to achieving your scientific goals.
Reference
- Chen H, et al. Regulated degradation of HMG CoA reductase requires conformational changes in sterol-sensing domain. Nature Communications. 2022, 13(1): 4273.