Structural Research of Cysteine Proteases
Cysteine proteases are a family of enzymes that play a significant role in cellular processes. These proteases have received considerable attention because of their involvement in a wide range of diseases, including cancer, arthritis, and neurodegenerative disorders. The structural research of cysteine proteases is essential for understanding their function and designing potential drugs to target these enzymes. Cysteine proteases contain a catalytic cysteine residue, and these enzymes have a conserved catalytic triad, which consists of cysteine, histidine, and asparagine. The catalytic cysteine residue plays a crucial role in the proteolysis process by forming a covalent bond with the substrate. The structural research of cysteine proteases has been focused on understanding the catalytic mechanism of these enzymes and their interactions with substrates and inhibitors.
Glycosylphosphatidylinositol (GPI) membrane anchors are complex glycophospholipids used to tether proteins to the cell surface. GPI transamidase (GPIT) is a complex enzyme composed of five subunits (PIGK, PIGU, PIGT, PIGS and GPAA1), each of which partially spans the endoplasmic reticulum (ER) membrane, extending into the lumen of the ER and other parts extending into the cytoplasm. It is the catalyst for attaching GPI to proteins, which belongs to the cysteine protease family C13 and is involved in the maturation of the GPI-anchored proteins.
A recent study has used single-particle cryo-EM to determine the structure of the human GPIT complex, revealing its overall assembly. Within this complex, the PIGK subunit acts as the catalytic component, and a C206-H164-N58 triad has been identified as crucial for the transamination reaction. Additionally, transmembrane helices create a cleft located beneath PIGK, which functions as a GPI substrate-binding site. The complex also features the presence of the ubiquitin E3 ligase RNF121 at the back, likely serving as a quality control factor for GPIT complex.
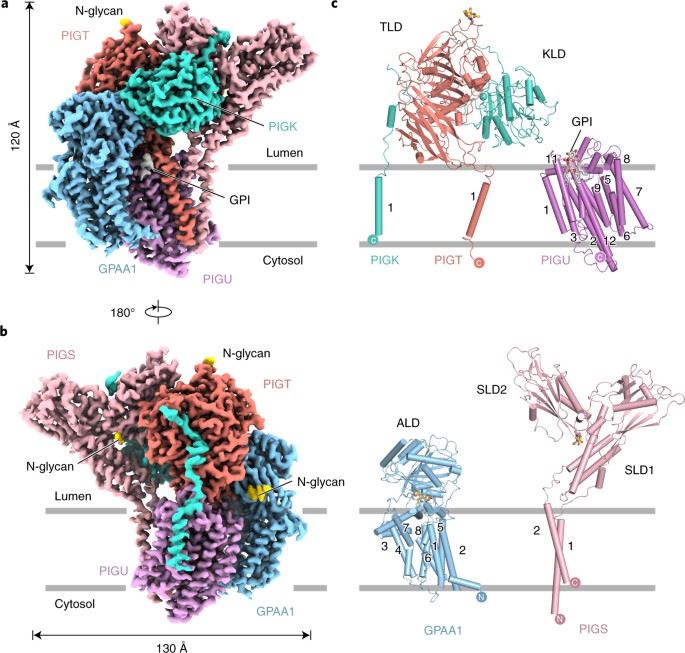 Figure 1. Architecture of human GPIT complex. (Zhang H, et al., 2022)
Figure 1. Architecture of human GPIT complex. (Zhang H, et al., 2022)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Glycosylphosphatidylinositol (GPI) transamidase (expressed in HEK293 cells) | Homo sapiens | Cryo-EM single particle analysis | 3.10 Å | 7W72 |
Table 1. Structural Research of Cysteine Proteases.
At Creative Biostructure, our team of scientists has extensive experience in protein crystallography, NMR spectroscopy, and cryo-electron microscopy. We have successfully solved many challenging protein structures, including cysteine proteases.
Using our X-ray crystallography service, we can determine the crystal structure of cysteine proteases and their complexes with inhibitors at atomic resolution. Our NMR spectroscopy service allows us to study the dynamic properties of cysteine proteases in solution, which is particularly useful for understanding their interactions with substrates and inhibitors. Our cryo-electron microscopy service provides high-resolution structural information on large macromolecular complexes. In addition to our structural analysis services, we also offer computational modeling and structure-based drug design services. If you are interested in studying the structure of cysteine proteases and want to learn more about our services, please don't hesitate to contact us.
Reference
- Zhang H, et al. Structure of human glycosylphosphatidylinositol transamidase. Nature Structural & Molecular Biology. 2022, 29(3): 203-209.
