Structural Research of Membrane-Integral Pyrophosphatases (M-PPases)
Membrane-integral pyrophosphatases (M-PPases) hydrolyze pyrophosphate in living organisms and use pyrophosphate as an energy source for sodium or proton pumping. The study of the M-PPases catalytic cycle is of great help to improve or hinder protein function. M-PPases play an important role in plant maturation and abiotic stress tolerance. Can be modified to improve plant survival. M-PPases are also a selective drug target that can be studied to reduce the virulence of common pathogens.
Research Progress on the Structure of M-PPases
In the past few years, there have been structural and functional data of M-PPases indicating the mechanism involving half-of-the-sites reactivity, exit channel motions and inter-subunit communication. M-PPases have a single transmembrane domain with 15-17 transmembrane (TM) helices. The helix forms two concentric rings around the four catalytic regions. There is a large hydrophilic region on the cytoplasmic side of the protein that extends into the cytoplasm about 20 Å.
By studying the structure of the M-PPases, the complete catalytic cycle of proton and sodium pumps can be understood, and the specificity of ion transport across membranes can also be explained. Further study on the structure of M-PPases by X-ray diffraction to understand the mechanism of their catalytic pathway.
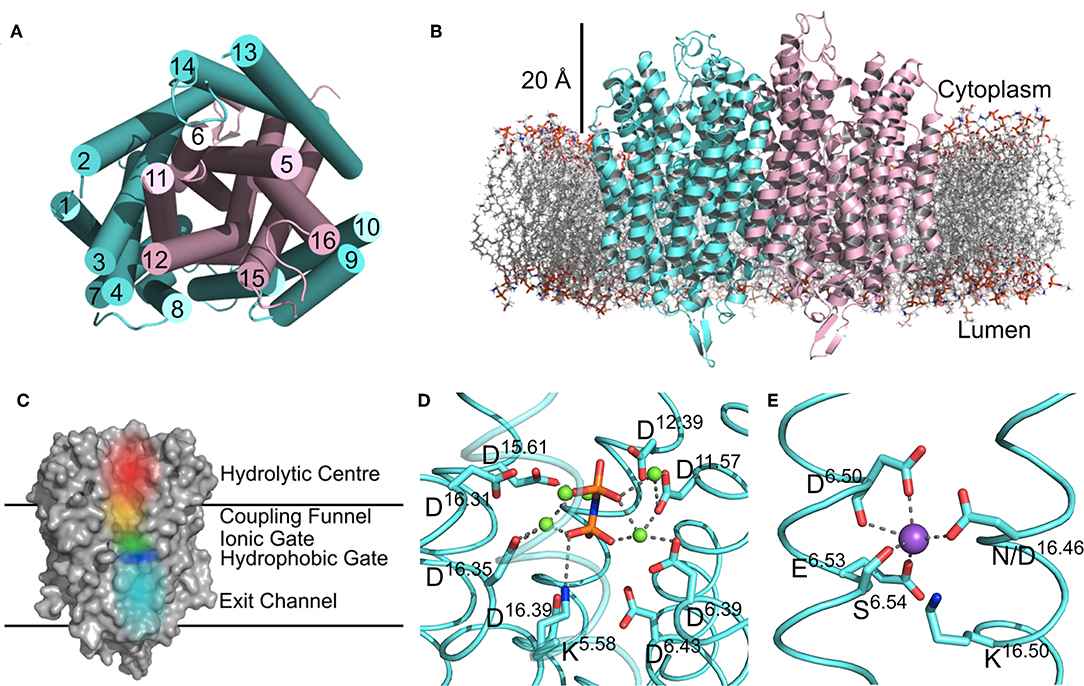 Figure 1. Structural features of M-PPases. (Holmes AOM et al., 2019)
Figure 1. Structural features of M-PPases. (Holmes AOM et al., 2019)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Proton-Pumping Pyrophosphatase | Vigna radiata var. radiata | X-ray diffraction | 3.50 Å | 5GPJ |
| Sodium pumping membrane integral pyrophosphatase in complex with imidodiphosphate and magnesium, and with bound sodium ion | Thermotoga maritima MSB8 | X-ray diffraction | 2.495 Å | 5LZQ |
| Thermotoga maritima sodium pumping membrane integral pyrophosphatase in complex with tungstate and magnesium | Thermotoga maritima MSB8 | X-ray diffraction | 4.00 Å | 5LZR |
| Thermotoga Maritima sodium pumping membrane integral pyrophosphatase with metal ions in active site | Thermotoga maritima | X-ray diffraction | 2.60 Å | 4AV3 |
| Membrane bound pyrophosphatase in complex with imidodiphosphate and N-[(2-amino-6-benzothiazolyl) methyl]-1H-indole-2-carboxamide (ATC) | Thermotoga maritima MSB8 | X-ray diffraction | 3.41 Å | 6QXA |
| H-Translocating Pyrophosphatase | Vigna radiata | X-ray diffraction | 2.35 Å | 4A01 |
| Proton pyrophosphatase - two phosphates-bound | Vigna radiata var. radiata | X-ray diffraction | 3.299 Å | 6AFS |
| Proton pyrophosphatase - E301Q | Vigna radiata var. radiata | X-ray diffraction | 2.492 Å | 6AFT |
| Proton pyrophosphatase-L555M mutant | Vigna radiata var. radiata | X-ray diffraction | 2.15 Å | 6AFU |
| Proton pyrophosphatase-L555K mutant | Vigna radiata var. radiata | X-ray diffraction | 2.701 Å | 6AFV |
| Proton pyrophosphatase-T228D mutant | Vigna radiata var. radiata | X-ray diffraction | 2.185 Å | 6AFW |
| Proton pyrophosphatase - E225A | Vigna radiata var. radiata | X-ray diffraction | 2.301 Å | 6AFX |
| Proton pyrophosphatase-E225S mutant | Vigna radiata var. radiata | X-ray diffraction | 2.401 Å | 6AFY |
| Proton pyrophosphatase-E225H mutant | Vigna radiata var. radiata | X-ray diffraction | 2.483 Å | 6AFZ |
Table 1. Structural research of membrane-integral pyrophosphatases (M-PPases).
In recent years, an increasing number of M-PPases structures have been analyzed by X-ray crystallography. To explore its important role in plant maturation and abiotic stress. As well as pharmacological modulation through structural studies to reduce common pathogen virulence.
If you want to study the M-PPases structure by X-ray diffraction. Creative Biostructure is your perfect solution. We have long been committed to the study of structural biology and membrane proteins, and have rich experience.
In addition to the structural determination of membrane proteins, we can also analyze nucleic acids, ribosomes, small proteins, protein complexes, protein-ligand complexes and viruses. If you are interested in our services, please contact us for more details.
References
- Kajander T, et al. Inorganic pyrophosphatases: one substrate, three mechanisms. FEBS Letters. 201, 587(13):1863-1869.
- Holmes AOM et al. The Function of Membrane Integral Pyrophosphatases From Whole Organism to Single Molecule. Frontiers in Molecular Biosciences. 2019; 6:132.
- Kellosalo J, et al. Crystallization and preliminary X-ray analysis of membrane-bound pyrophosphatases. Mol Membr Biol. 2013, 30(1):64-74.