Structural Biology of Viruses
A virus is a non-cellular organism in which genetic material is surrounded by a protective protein shell. Some viruses have more complex structures, and the nucleic acids of these viruses are encapsulated in an enveloped virus. The virus relies on the host for the synthesis of new proteins. Generally, a type of virus infects only one type of host, but some viruses can infect various living cells including bacteria, archaea, fungi, plants, and animals. Viruses have different sizes and shapes. The genetic material of a virus can be either DNA or RNA. Viral nucleic acids can be single-stranded or double-stranded, and the viral genome can be divided into one or more fragments, encoding as few as four or five proteins, and as many as hundreds of proteins. The structural proteins of the virus can make up virions that can infect host cells, while the nonstructural proteins are responsible for the efficient assembly of virions. For example, viruses that make up genomes from RNA can encode an enzyme that catalyzes the replication of their genetic material, since host cells usually do not contain enzymes with similar functions.
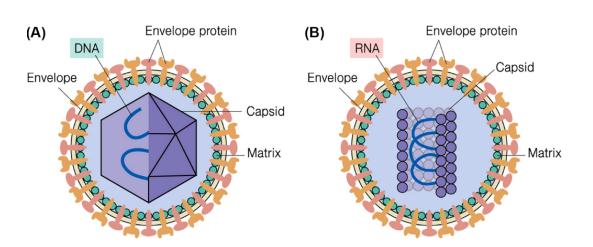 Figure 1. Schematic diagram of virus particles. (A) A typical envelope virus particle with a spherical capsid (B) A typical envelope virus particle with a helical capsid.
Figure 1. Schematic diagram of virus particles. (A) A typical envelope virus particle with a spherical capsid (B) A typical envelope virus particle with a helical capsid.
- Viral genome structure
- Capsid structure
- Envelope structure and function
- Virus-like particles
- Virus structure research
Viral genome structure
As in cells, the genetic information carried by the nucleic acids of each virus encodes all proteins. Although the genome of all known cells consists of double-stranded DNA, the genome of the virus may consist of single-stranded or double-stranded DNA or RNA. The size of the viral genome can vary widely, from about 5-10 kb to more than 100-200 kb. Only a few groups of viruses use DNA. Most viruses use single-stranded RNA to maintain all their genetic information. The known structure of the viral genome is outlined below.
- DNA viral genome structure
Most DNA viruses contain a single genome of linear double-stranded DNA (dsDNA). However, some viruses have a circular dsDNA genome, such as papovaviruses. The dsDNA acts as a template for mRNA and self-transcription. Linear single-stranded DNA (ssDNA) is found in the members of the Parvovirus family. Circular ssDNA is found in the members of the Circovirus family which comprise the smallest autonomously propagated viruses.
- RNA viral genome structure
About 70% of the viruses are RNA viruses, and they have significant differences in genomic structure. Due to the error rate of the enzymes involved in RNA replication, these viruses generally show higher mutation rates than DNA viruses, making them highly adaptable to new hosts. Viral RNA can be single-stranded RNA (ssRNA) or double-stranded RNA (dsRNA). In addition, there are two types of ssRNA viruses, (+) ssRNA viruses and (−) ssRNA viruses. In most instances, genomic RNA is a positive (or sense) strand, because it can directly translate viral proteins as messenger RNA (mRNA). However, a small number of RNA viruses have a negative (or antisense) strand, which is a complementary strand to the positive strand. In this case, the virus has an enzyme called RNA-dependent RNA polymerase (reverse transcriptase), which must first catalyze the production of complementary mRNA from viral genomic RNA before viral protein synthesis may occur. Additionally, the genome of some RNA viruses is segmented, which means that a virion contains several different RNA molecules, like different chromosomes.
Capsid structure
The capsid is a protein shell that encapsulates the viral genome and is a necessary component of the structure and function of the virus. The capsid protects the genomic material from physical and chemical destructors. A nucleic acid molecule cannot encode a single protein enough to encapsulate the nucleic acid molecule, so the viral protein capsid is assembled from multiple identical protein molecules. To protect the viral genome, a very stable capsid is required. To do this, the protein assembly of the viral capsid is arranged symmetrically, and the interaction between the different subunits depends on the same protein-protein interaction surface. Two kinds of capsid structures have been found in viruses, spherical capsids and helical capsids (filamentous and rod-shaped), respectively corresponding to two essentially different symmetries, icosahedral symmetry, and helical symmetry. The icosahedral capsid has 20 facets, each of which is triangular. The T number defines the topological characteristics of the icosahedral structure. It indicates the number of subunits that constitute a triangular facet made of three fivefold axes of symmetry. It means that the number of subunits constitutes a triangular face consisting of three five-fold symmetry axes. Some viruses have more complex shapes, for instance, phage T4 has an icosahedral head and a helical tail, and protein complexes making up pilus and other protein structures that are important for the infection process on the head and tail.
Envelope structure and function
Viral particles can be either "enveloped" or "nonenveloped", depending on the presence or absence of the envelope. In some animal viruses, there are lipid bilayer membranes around the nucleic acid and capsid. The lipid bilayer membrane is encapsulated when the virus leaves the host by budding, and the membrane carries virus-encoded proteins that are involved in the formation of the outer surface of the virion. These viral proteins have their biological functions, such as binding to host cell receptors or forming channels in viral membrane, which play a role in membrane fusion and cell entry. Many encapsulated viruses also contain matrix proteins that coat the inner leaf of the lipid bilayer, and these internal proteins link the nucleocapsid to the envelope. Some enveloped viruses can change shape due to the flexibility of the outer membrane. The mechanism by which the virus enters the host cell depends on the type of protective coat: the enveloped virus can infect the host by fusing the outer membrane of the virion itself into the host cell membrane, while the nonenveloped virus can only enter the host cell by other mechanisms.
Virus-like particles
It is well known that virus-like particles (VLPs) are viral empty shells without a genome. VLPs share the same virion structural properties and are incapable of replication, making VLPs to be considered efficient and safe candidates for vaccine platforms and delivery systems. As several VLP-based vaccines have been approved in recent years, the application value of VLPs becomes more acceptable and shows more potential in a broad range of areas.
Virus structure research
Viral particles represent "nanoparticles" found in nature, most of which range from 30 to 300 nm and can only be observed by electron microscopy. Therefore, optical instruments are essential for examining the morphology of viral particles. Three modern techniques can be used to visualize viral particles, namely electron microscopy (EM), cryo-electron microscopy (Cryo-EM), and X-ray crystallography. Structural virology tools will make a greater contribution to advancing our knowledge of many aspects of virology and ultimately help us design better strategies to fight against pathogens.
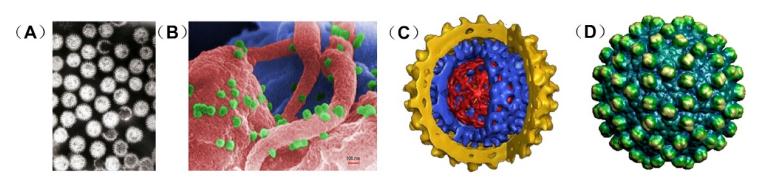 Figure 2. Tools for virus structure research. (A) Transmission electron micrograph of rotavirus particles (B) Scanning electron microscope image of HIV particles (green) budding from the infected T lymphocytes (pink and blue) (C) High-resolution image of a hepatitis B virus obtained by cryo-electron microscope (D) Hepatitis B virus capsid structure obtained by X-ray crystallography.
Figure 2. Tools for virus structure research. (A) Transmission electron micrograph of rotavirus particles (B) Scanning electron microscope image of HIV particles (green) budding from the infected T lymphocytes (pink and blue) (C) High-resolution image of a hepatitis B virus obtained by cryo-electron microscope (D) Hepatitis B virus capsid structure obtained by X-ray crystallography.
| Virus Family | Description |
| Alphaflexiviridae | Contains viruses with flexuous filamentous virions that infect plants and a few viruses discovered in plant-infecting fungi. |
| Arenaviridae | Members are generally associated with rodent-transmitted diseases in humans. |
| Arteriviridae | Includes equine arteritis virus (EAV; the family prototype), porcine reproductive and respiratory syndrome virus genotypes I and II (PRRSV-I and PRRSV-II), lactate dehydrogenase-elevating virus (LDV), and simian hemorrhagic fever virus (SHFV). |
| Birnaviridae | Comprises four genera (Aquabirnavirus, Avibirnavirus, Blosnavirus, and Entomobirnavirus) of nonenveloped viruses whose genome consists of two segments (segments A and B) of dsRNA. |
| Bromoviridae | Member genera are Alfamovirus, Anulavirus, Bromovirus, Cucumovirus, Ilarvirus, and Oleavirus. |
| Bunyaviridae | The largest virus family, with more than 350 member viruses included in 5 genera: Orthobunyavirus, Hantavirus, Nairovirus, Phlebovirus, and Tospovirus. |
| Caliciviridae | Member genera are Vesivirus, Lagovirus, Norovirus, Sapovirus, and Nebovirus, diseases associated with this family include feline calicivirus (respiratory disease), rabbit hemorrhagic disease virus (often-fatal hemorrhages), and Norwalk group of viruses (gastroenteritis). |
| Carmotetraviridae | There is currently only one genus in this family, Alphacarmotetravirus, and one species in this genus, the type species Providence virus, Lepidopteran insects serve as natural hosts. |
| Circoviridae | Member genera are Circovirus and Gyrovirus, which are unenveloped and have circular ssDNA genomes. |
| Coronaviridae | Enveloped, positive-stranded RNA viruses, severe acute respiratory syndrome-associated CoV (SARS-CoV), and Middle East respiratory syndrome CoV (MERS-CoV) belong to this family. |
| Dicistroviridae | There are 15 species in this family, non-enveloped, with icosahedral geometries. |
| Filoviridae | Two members of the family that are commonly known are the Ebola virus and the Marburg virus. |
| Flaviviridae | Arthropod-borne, enveloped, RNA viruses, including four genera (Flavivirus, Pestivirus, Hepacivirus, and Pegivirus), contain many important human pathogens, such as Yellow fever virus (YFV), Dengue fever virus (DFV), Zika virus, and Hepatitis C virus (HCV). |
| Hepadnaviridae | Reverse transcribing viruses, the prototype virus of the family is the hepatitis B virus. |
| Hepeviridae | Includes two genera: the genus Orthohepevirus with four separate species that include the Hepatitis E virus (HEV) and the genus Piscihepevirus with a single virus species from fish. |
| Herpesviridae | Large, enveloped viruses that possess a linear dsDNA, eight human herpesviruses were discovered, which are subdivided into three genera: α-, β-, and γ-herpesvirus. |
| Leviviridae | RNA phage, are comprised of phages fr, f2, ms2, Qβ, GA, JP34, PRR1, and PP7. |
| Matonaviridae | A family of small, enveloped viruses with (+) ssRNA genomes, represented by the Rubella virus. |
| Nodaviridae | Members of the family have small, nonenveloped, icosahedral virions, diseases associated with this family include viral encephalopathy and retinopathy in fishes. |
| Orthomyxoviridae | Includes viruses with genomes composed of several segments (6-8) of ssRNA, the most important members of the family are the influenza viruses. |
| Papillomaviridae | Non-enveloped DNA viruses, infections by a subset of human papillomaviruses (HPVs), known as high-risk types, can lead to cancer. |
| Paramyxoviridae | Member genera include Rubulavirus, Avulavirus, Respirovirus, Pneumovirus, and Metapneumovirus, diseases associated with this (-) ssRNA virus family include measles, mumps, and respiratory tract infections. |
| Parvoviridae | Small icosahedral, nonenveloped animal viruses, Canine parvovirus causes a fatal and contagious disease in dogs, a parvovirus causes feline distemper in cats. |
| Picornaviridae | Member genera include Enterovirus, Cardiovirus, Aphthovirus, Hepatovirus, and Parechovirus, the viruses in this family can cause a range of diseases including paralysis, meningitis, hepatitis, and poliomyelitis. |
| Pneumoviridae | A new virus family comprises two genera Metapneumovirus and Orthopneumovirus. |
| Polyomaviridae | Some members of the family are oncoviruses, meaning they can cause tumors. |
| Potyviridae | Plant-infecting (+) ssRNA viruses, many of which are of great agricultural significance. |
| Reoviridae | Represents the largest family of dsRNA viruses, Reoviruses can affect the gastrointestinal system (such as Rotavirus) and respiratory tract. |
| Retroviridae | Exploits reverse transcription of viral RNA into DNA during replication, members include human immunodeficiency virus (HIV), feline leukemia, and several cancer-causing viruses. |
| Rhabdoviridae | Member genera are Vesiculovirus, Lyssavirus, Ephemerovirus, Novirhabdovirus, Cytorhabdovirus, and Nucleorhabdovirus, Rabies virus belongs to the family. |
| Secoviridae | Non-enveloped, with icosahedral geometries, plants serve as natural hosts. |
| Togaviridae | Includes two genera, Alphavirus and Rubivirus, contains the significant human pathogens eastern equine encephalitis, western equine encephalitis, Sindbis virus, Chikungunya virus, and Mayaro virus. |
| Tombusviridae | A family of (+) ssRNA plant viruses. |
| Tymoviridae | A family of (+) ssRNA plant viruses. |
Table 1. Virus family.
| Structural Research of Virus | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||
Creative Biostructure is specialized in the field of structural biology, and we provide contract services for viral particle identification and characterization.
References
- Ryu W S. Chapter 2 – Virus Structure. Molecular Virology of Human Pathogenic Viruses. 2017: 21-29.
- Gelderblom H R. Structure and classification of viruses. Medical Microbiology. 4th edition, 1996.