Structural Research of Omp85-TpsB Outer Membrane Transporter Superfamily
The Omp85/TPS superfamily is a β-barrel protein found only in Gram-negative bacteria, chloroplasts, and mitochondrial outer membrane (OM). These proteins are called Toc75 in chloroplasts and Sam50 or Tob55 in mitochondria. These proteins catalyze beta-barrel protein insertion into the membrane and transmembrane translocation of the protein. Omp85/TPS family members play an important role in energy-dependent transport, protein secretion and input, protein automatic transport, lipid transport, and other physiological activities.
Progress in structural studies of the Omp85/TPS superfamily
Omp85/TPS proteins contain an amphiphilic β-folding layer, which forms a closed β-barrel structure and serves as a transmembrane structural domain. β-Barrels are 8-36 β-strands. The N-terminal part of the Omp85 superfamily consists of 1-7 repeats of soluble polypeptide transporter-associated (POTRA) structural domains with two α-helices and three β-folds. The C-terminus has a 16-stranded β-barrel pore that is highly conserved.
Action mechanism of the Omp85 protein BamA in bacteria
BamA is a highly conserved Omp85 protein in bacteria. It is an important component of the barrel assembly mechanism (BAM) that promotes the integration of beta-barrel proteins into OM. BamA secretes the exogenous protein TpsA. The TpsA protein is a virulence factor that folds into an extended beta-helix and is encoded in the same manipulator as its homologous TpsB transporter.
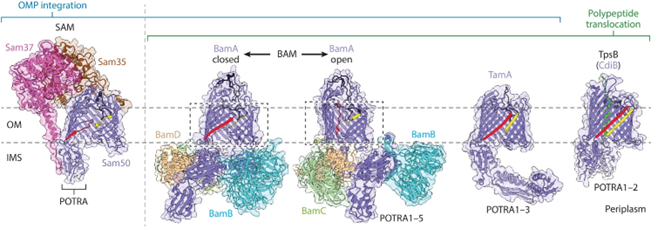 Figure 1. Structure of Omp85 protein superfamily members. (Doyle MT, et al., 2022)
Figure 1. Structure of Omp85 protein superfamily members. (Doyle MT, et al., 2022)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| BamA | Neisseria gonorrhoeae FA 1090 | X-ray diffraction | 3.2 Å | 4K3B |
| Outer membrane protein insertase BamA with one POTRA domain. | Escherichia coli | X-ray diffraction | 3 Å | 4C4V |
| BamA lacking POTRA domains 1-3 | [Haemophilus] ducreyi | X-ray diffraction | 2.913 Å | 4K3C |
| BamA-mediate Outer Membrane Protein Biogenesis | Escherichia coli K-12 | X-ray diffraction | 2.604 Å | 4N75 |
| BamB and BamA P3-5 complex | Escherichia coli K-12 | X-ray diffraction | 2.15 Å | 4XGA |
| A membrane complex | Escherichia coli K-12 | X-ray diffraction | 3.555 Å | 5AYW |
| BamABCDE complex, outer membrane beta-barrel assembly machinery entire complex | Escherichia coli | X-ray diffraction | 2.9 Å | 5D0O |
| BamACDE complex, outer membrane beta-barrel assembly machinery (BAM) complex | Escherichia coli | X-ray diffraction | 3.5 Å | 5D0Q |
| BamACDE subcomplex | Escherichia coli | X-ray diffraction | 3.392 Å | 5EKQ |
| BamA | Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 | X-ray diffraction | 2.92 Å | 5OR1 |
| BamA beta-barrel with a C-terminal extension | Escherichia coli O157:H7 | X-ray diffraction | 2.6 Å | 6FSU |
| BamA POTRA4 | Haemophilus influenzae | X-ray diffraction | 2.03 Å | 6IZS |
| BamA POTRA3-5 | Haemophilus influenzae | X-ray diffraction | 2.03 Å | 6IZT |
| BamA POTRA1-4 | Haemophilus influenzae | X-ray diffraction | 3 Å | 6J09 |
| BAM complex | Escherichia coli K-12 | X-ray diffraction | 3.19 Å | 6LYQ |
| BAM complex | Escherichia coli K-12 | X-ray diffraction | 3.28 Å | 6LYR |
| BAM complex | Escherichia coli K-12 | X-ray diffraction | 3.05 Å | 6LYS |
| BAM complex | Escherichia coli K-12 | X-ray diffraction | 4.2 Å | 6LYU |
| BamA beta-barrel in complex with nanobody E6 | Escherichia coli O157:H7 | X-ray diffraction | 1.938 Å | 6QGW |
| BamABCDE in MSP1D1 nanodisc | Escherichia coli | Cryo-EM single particle analysis | 6.65 Å | 6SMX |
| BamABCDE in MSP1D1 nanodisc ensemble 0-1 | Escherichia coli | Cryo-EM single particle analysis | 10.8 Å | 6SN0 |
| BamABCDE in MSP1D1 nanodisc ensemble 0-2 | Escherichia coli | Cryo-EM single particle analysis | 9.5 Å | 6SN2 |
| BamABCDE in MSP1D1 nanodisc ensemble 1-2 | Escherichia coli | Cryo-EM single particle analysis | 10.5 Å | 6SO7 |
| R450A mutant of the membrane protein FhaC | Bordetella pertussis | X-ray diffraction | 3.5 Å | 3NJT |
| Membrane Transporter FhaC | Bordetella pertussis Tohama I | X-ray diffraction | 2.9 Å | 4QKY |
Table 1. Structural research of mp85-TpsB outer membrane transporter superfamily.
Creative Biostructure has a long-standing commitment to structural biology and membrane protein research. We have advanced equipment and experienced experts in membrane protein structure determination. We provide our clients with protein structural analysis services using NMR spectroscopy, X-ray crystallography and cryo-electron microscopy (cryo-EM) that help to further explore the function of proteins. If you are interested in our services, please contact us for more details.
References
- Doyle MT, et al. Function of the Omp85 Superfamily of Outer Membrane Protein Assembly Factors and Polypeptide Transporters. Annu Rev Microbiol. 2022. 76:259-279.
- Simmerman RF, et al. Structure and function of POTRA domains of Omp85/TPS superfamily. Int Rev Cell Mol Biol. 2014. 308:1-34.