Structural Research of Bovine Papillomavirus
Bovine papillomaviruses (BPV) are a paraphyletic group of DNA viruses in cattle that belong to the family Papillomaviridae, capable of inducing benign cutaneous or mucosal epithelial lesions. Generally, benign tumors affecting the skin or mucous membranes resolve spontaneously, but in exceptional cases, the defense system may become overwhelmed, leading to cancer and serious economic losses. The mechanism by which BPV interacts with the host is unknown. Therefore, researchers have assessed the interaction of BPV variants with markers and receptors involved in squamous cell carcinoma through three-dimensional structural analysis, providing valuable insights for the development of novel therapeutic strategies against BPV-associated diseases.
Structural Features of BPV
The structure of BPV is determined by cryo-electron microscopy (cryo-EM) with the resolution of 3.6 Å. Like other papillomaviruses, BPVs are small, non-enveloped viruses with an icosahedral capsid of about 50-60nm. The capsid consists of the L1 and L2 structural proteins. All BPVs have a circular double-stranded DNA genome of 7.3-8.0 kb. The open reading frames (ORFs) are all located on one strand and are divided into early, late, and a non-coding long control region (LCR). The early region encodes proteins that contribute to viral replication (E1, E2, and E4) and oncoproteins (E5, E6, and E7). The late region encodes the structural proteins L1 and L2. Densitograms obtained by single-particle analysis show traces of the L1 polypeptide chain and reveal that the N-terminal and C-terminal "arms" of the subunit (extending from its β-jelly roll core) are linked to neighboring pentamers.
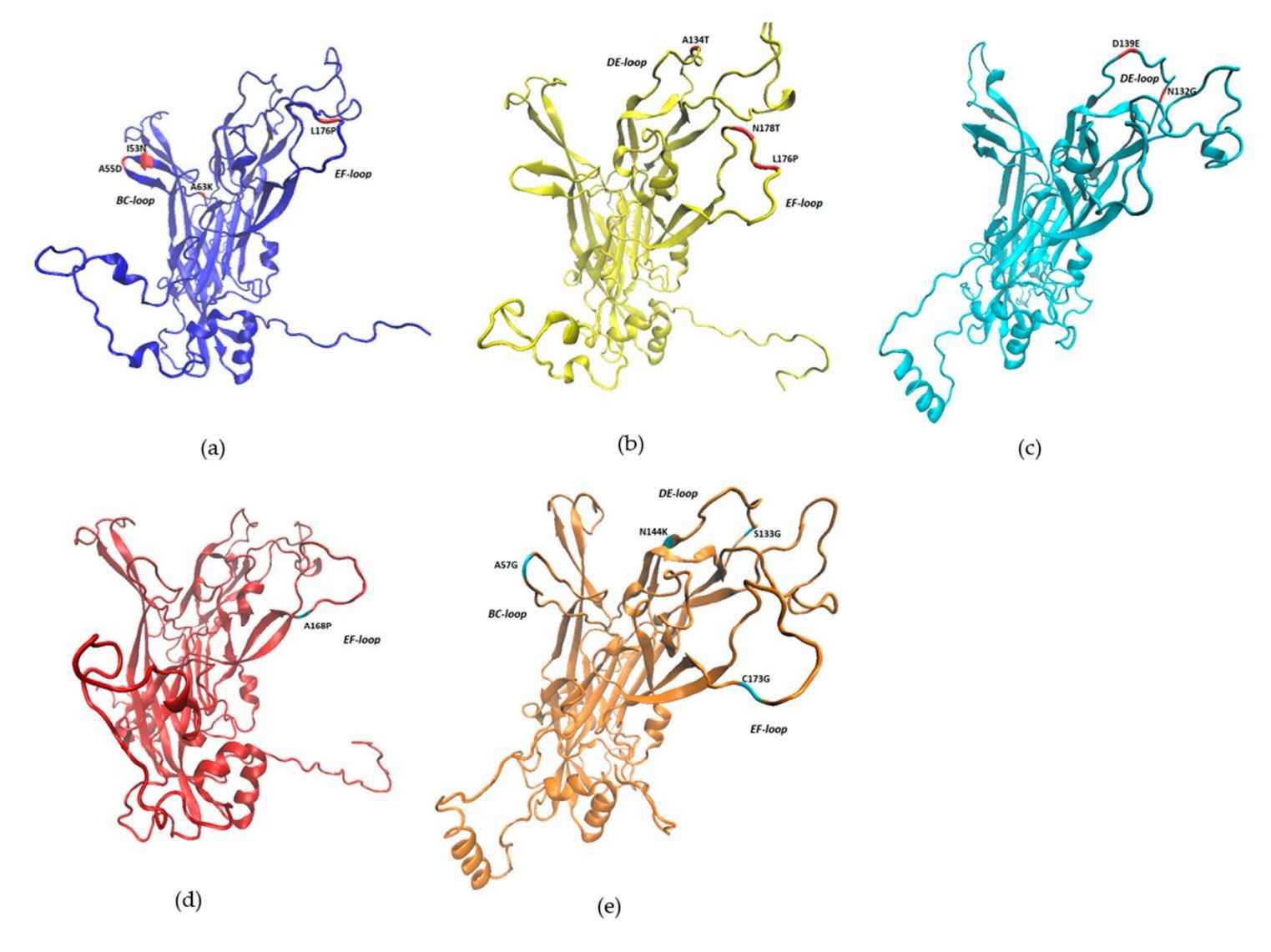 Figure 1. The 3D structures of BPV L1 protein. (Figueirêdo RP, et al., 2020)
Figure 1. The 3D structures of BPV L1 protein. (Figueirêdo RP, et al., 2020)
Vaccine Development for BPV
Structural research on BPV has facilitated the development of vaccines and antiviral strategies. By determining the structure of viral capsid proteins and recognizing immunogenic epitopes, researchers have been able to design vaccines capable of triggering specific immune responses against BPV. In addition, structural characterization of viral enzymes and protein-protein interactions provide targets for the development of antiviral drugs and therapeutic interventions. Currently, researchers have developed vaccines against BPV types 1, 2, and 4. Prophylactic vaccination is performed with the intact virus, virus-like particles (L1 or L1+L2), L1 proteins, or L2 proteins. The proteins provide durable protection against the same BPV type of attack. Therapeutic vaccination with BPV-4 E7 or BPV-2 L2 induces early wart regression. In addition, these vaccine systems have served as models for the successful development of prophylactic vaccines against HPV associated with cervical and anal cancers.
 Figure 2. Design of polyionic BPV L1 protein VLP vaccine. (Viscidi RP, et al., 2023)
Figure 2. Design of polyionic BPV L1 protein VLP vaccine. (Viscidi RP, et al., 2023)
As a leader in viral structural biology. Creative Biostructure provides customized virus structure analysis services and high-quality virus-like particles (VLPs) products to help customers develop BPV vaccines or antiviral drugs, and formulate related prevention and treatment strategies.
| Cat No. | Product Name | Virus Family | Source | Composition |
| CBS-V552 | Bovine papillomavirus type 1 VLP (L2 Proteins) | Papillomaviridae | Insect cell recombinant | L2 |
| CBS-V553 | Bovine papillomavirus 1 VLP (L1 Proteins) | Papillomaviridae | E. coli recombinant | L1 |
| CBS-V785 | Bovine papillomavirus VLP (L1; L2; GFP Proteins) | Papillomaviridae | Insect cell recombinant | L1; L2; GFP |
| Explore All Bovine Papillomavirus VLP Products | ||||
Creative Biostructure is committed to providing excellent cryo-EM services, which are effective methods for studying the three-dimensional structure of virus particles. We use advanced testing equipment and methods to offer the best customer service and results. Our laboratory staff have undergone extensive training and certification, possessing professional knowledge and proficiency to ensure accurate and reliable data delivery. Please contact us to obtain a formal quote.
References
- Figueirêdo RP, et al. High Genotypic Diversity, Putative New Types and Intra-Genotype Variants of Bovine Papillomavirus in Northeast Brazil. Pathogens. 2020. 9(9): 748.
- Viscidi RP, et al. Bioengineered Bovine Papillomavirus L1 Protein Virus-like Particle (VLP) Vaccines for Enhanced Induction of CD8 T Cell Responses through Cross-Priming. International Journal of Molecular Sciences. 2023. 24(12): 9851.
- Bocaneti F, et al. Bovine Papillomavirus: New Insights into an Old Disease. Transbound Emerg Dis. 2016. 63(1): 14-23.
- Wolf M, et al. Subunit interactions in bovine papillomavirus. Proc Natl Acad Sci U S A. 2010. 107(14): 6298-6303.