Structural Research of Birnaviridae
Birnaviridae consists of four genera with a single protein coat and double-stranded RNA (dsRNA) genomes. Birds, fish, mollusks, and insects are their natural hosts. Of these, infectious pancreatic necrosis virus (IPNV) in salmonids and infectious bursal disease virus (IBDV) in pheasants have a significant economic impact on the aquaculture and poultry industries, respectively. Recent research has revealed that the capsid of dual RNA viruses is mainly composed of VP2 proteins with a great antigenic propensity. Structural resolution of dual RNA viruses is essential for the timely detection of viruses in infected animals and vaccine development.
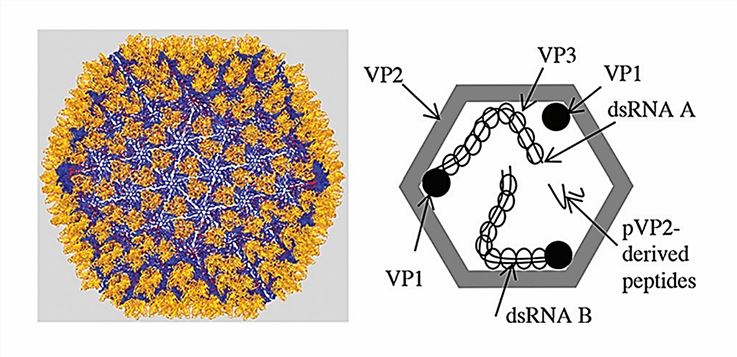 Figure 1. Structure of IBDV particles. (Delmas B, et al., 2019)
Figure 1. Structure of IBDV particles. (Delmas B, et al., 2019)
The Overall Structure of Birnaviridae
The genome of Birnaviridae consists of two dsRNA segments (segments A and B), and the viral particles are unenveloped icosahedral particles with a triangular section T = 13 and a diameter of approximately 700 Å. The A fragment encodes a polyprotein that is co-translated by the VP4 protease to form pre-(p-) VP2, VP3, and VP4. The A fragment also contains an overlapping secondary open reading frame (ORF) encoding VP5, which may play a role in regulating virulence and non-cleavage export. The B fragment encodes VP1, the viral RNA-dependent RNA polymerase (RdRP). Within the viral particle, the dual RNA viral genome fragment exists as a viral ribonucleoprotein (vRNP) complex. The VP3 binds to the inner surface of the VP2 capsid. VP1 exists in free form within the capsid and is attached to the 5' end of the genome strand as VPg.
Advances in the Research of Birnaviridae
Currently, most of the research on dual RNA viruses has focused on IBDV. The X-ray crystallography studies of IBDV virus particles have shown that the surface of the capsid consists of pentamers and hexamers of the VP2 homotrimer. A highly variable region (HVR) containing four hydrophilic loops containing the major epitopes of the anti-IBDV antibody is protruding from its exterior. Like other dsRNA viruses, birnavirus particles are transcriptionally active in the presence of nucleotides, extruding non-polyadenylated mRNA through pores in the capsid.
Creative Biostructure is the world's leading supplier of virus-like particles (VLPs). With our cutting-edge research and expertise in the field of virology, we are committed to providing innovative solutions for Birnaviridae-related applications.
Our comprehensive range of Birnaviridae VLP products is customized for resolving their unique structures. By analyzing the structures of the various components of Birnaviridae, it is possible to aid in developing antiviral drugs and vaccines against these viruses, develop effective control strategies, and improve animal health in the poultry industry.
| Cat No. | Product Name | Virus Name | Source | Composition |
| CBS-V658 | Infectious Bursal Disease Virus VLP (VP2 Proteins) | Infectious bursal disease virus | Yeast recombinant | VP2 |
| Explore All Birnaviridae Virus-like Particle Products | ||||
Creative Biostructure supports researchers in finding the best solutions for virus particle identification and characterization. Our experienced scientists are skilled in the use of cryo-electron microscopy (cryo-EM) to reveal the structure of Birnaviridae.
We offer off-the-shelf VLP products for worldwide delivery. If we do not have the VLP product you need in our current catalog, please feel free to contact us. In addition, we offer custom VLP construction services to satisfy individual clients' requirements.
References
- Delmas B, et al. ICTV virus taxonomy profile: Birnaviridae. J Gen Virol. 2019. 100(1): 5-6.
- Brodrick AJ, Broadbent AJ. The Formation and Function of Birnaviridae Virus Factories. Int J Mol Sci. 2023. 24(10): 8471.
- Coulibaly F, et al. The birnavirus crystal structure reveals structural relationships among icosahedral viruses. Cell. 2005. 120(6): 761-772.