Structural Research of Hepadnaviridae
The Hepadnaviridae consist of small enveloped viruses with a 3.0-3.4kb partial double-stranded DNA genome, for which humans, apes, and birds are natural hosts. All members of the family express three sets of proteins (pre-C/C, polymerase, and pre-S/S). Hepatitis B virus (HBV), a typical member of the Hepadnaviridae, replicates through reverse transcription of RNA intermediates in hepatocytes and causes hepatitis B infection, which leads to a serious public health problem. Therefore, a deeper understanding of the structure of Hepadnaviridae members has theoretical and practical implications for research on molecular mechanisms and the discovery of antiviral drugs or vaccines.
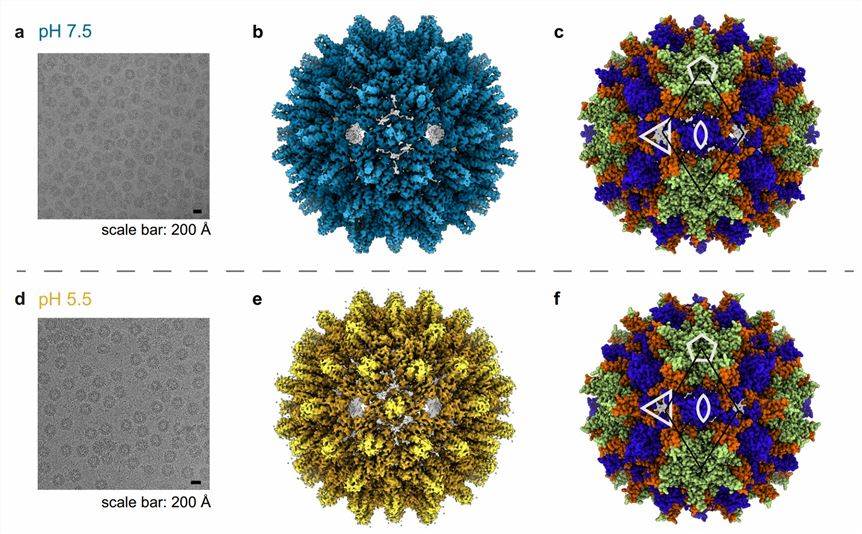 Figure 1. The cryo-EM micrographs of the Hepadnaviridae ACNDV capsid. (Pfister S, et al., 2023)
Figure 1. The cryo-EM micrographs of the Hepadnaviridae ACNDV capsid. (Pfister S, et al., 2023)
Overall Structure of Hepadnaviridae Viral Particles
Hepadnaviridae are mostly spherical and contain two or three surface proteins in the envelope to induce protective immunity. The icosahedral nucleocapsid contains part of the double-stranded DNA genome, DNA polymerase, protein kinase, and molecular chaperones. The core proteins expressed in bacteria assemble into icosahedral particles that closely resemble those found in infected livers. Two sizes of particles containing 180 (T = 3) or 240 (T = 4) copies of the core protein aggregate into dimers that produce 90 or 120 spikes on the shell surface.
Progress in the Structure Research on HBV Capsid Protein
HBV viral particles consist of a structurally unique capsid (core antigen), which contains the genome and polymerase and is surrounded by a lipid envelope embedded in antigenic proteins. The structure of the capsid protein (Cp) has been extensively researched by cryo-electron microscopy (cryo-EM), X-ray crystallography, and NMR spectroscopy. The structure indicates that the coat consists of a 183-residue polypeptide, of which the first 140 residues (N-terminal assembly structural domain NTD) carry out coat assembly. The NTD is a full α-helix fold in which the two long central helices form inversely parallel hairpins. The hairpins of the two Cp monomers combine into a four-helix bundle to form a stable dimer that serves as the basic building block of the icosahedral capsid.
As a leading company in the field of structural analysis of biological macromolecules, Creative Biostructure specializes in viral structure analysis services and the provision of virus-like particles (VLPs) products. We have an unwavering commitment to quality and adhere to stringent quality control procedures during the construction of our products to provide the highest quality virus-like particles. Our VLP products can be used for drug and antibody discovery and characterization and have been integrated into a variety of existing platforms.
| Cat No. | Product Name | Virus Name | Source | Composition |
| CBS-V073 | HBV VLP | Hepatitis B virus | Yeast recombinant | Surface antigen |
| CBS-V590 | HBV VLP (core Proteins) | Hepatitis B virus | E. coli recombinant | core |
| CBS-V595 | HBV VLP (Surface antigen Proteins) | Hepatitis B virus | Plant recombinant | Surface antigen |
| Explore All Hepadnaviridae Virus-like Particle Products | ||||
Creative Biostructure, a leading company in structural biology, has a team of highly skilled scientists with extensive expertise in the structural analysis of biomolecules, particularly in the area of viral structures. Their deep understanding of virology and cutting-edge technologies enables us to provide precise and comprehensive structural insights to our clients.
In addition, we have advanced tools such as cryo-electron microscopy (cryo-EM) and imaging systems capable of capturing high-resolution data and revealing the complex structure of viruses in exceptional detail. If you are interested in our services and products, please contact us for more details. We aim to be your trusted and reliable partner in the field of virus structure analysis and virus-like particles (VLPs) products.
References
- Pfister S, et al. Structural conservation of HBV-like capsid proteins over hundreds of millions of years despite the shift from non-enveloped to enveloped life-style. Nat Commun. 2023. 14(1): 1574.
- Magnius L, et al. ICTV Virus Taxonomy Profile: Hepadnaviridae. J Gen Virol. 2020. 101(6): 571-572.
- Roseman AM, et al. A structural model for maturation of the hepatitis B virus core. Proc Natl Acad Sci U S A. 2005. 102(44): 15821-15826.