Structural Research of Erythropoietin-Producing Hepatocellular Receptors
Erythropoietin-producing hepatocellular receptors (Eph) are a family of receptor tyrosine kinases that play important roles in the regulation of cell shape, movements, and attachment. Understanding the structural properties of these receptors is essential for developing targeted therapeutics for various diseases. Structural analysis of Eph receptors is a highly active research field. A high-resolution structure of the right-handed homodimeric transmembrane (TM) domain of the EphA1 was obtained in a membrane mimicking environment. This structure revealed a pH-induced realignment of the helix packing in the dimer between two characteristic dimerization motifs. Multiple characteristic dimerization motifs in the TM domain sequence of the Eph receptor suggest that its TM domains can associate diversely in the plasma membrane. This diversity allows the Eph receptor to participate in both ligand-independent and ligand-induced dimerization and clustering.
In another study, the high-resolution NMR structure of the left-handed homodimeric TM domain of EphA2 was investigated by embedding it into lipid bicelles. This study indicates that TM domains of the Eph receptors can self-associate in a different manner, demonstrating the diversity of TM domain formation within the same family of receptor protein kinases. Furthermore, this finding implies the so-called rotation-coupled activation mechanism of the receptor. Notably, EphA2 is the first single-spanning membrane receptor with a noncovalent left-handed dimeric structure of the TM domain that has been experimentally determined.
NMR spectroscopy technique is crucial in the structural analysis of complex protein systems like the Eph receptors. High-resolution structural studies using NMR spectroscopy provide valuable insights into the molecular details of the protein systems. Additionally, NMR can investigate dynamic structural changes, making it a powerful tool for studying protein-protein interactions, signal transduction, and drug discovery.
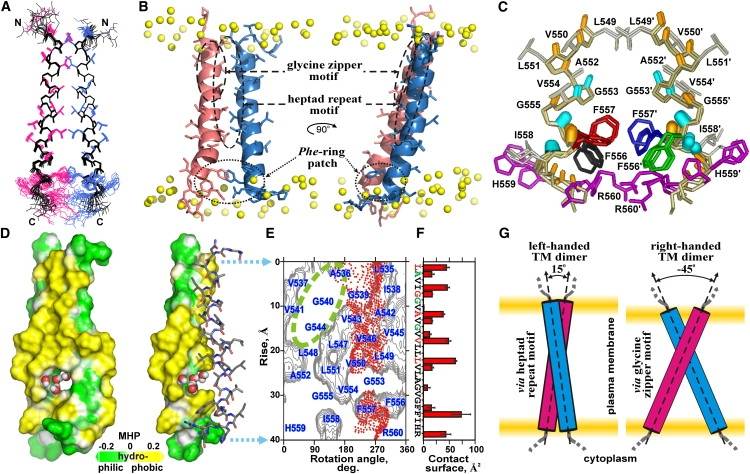 Figure 1. Spatial structure of the EphA2tm dimer. (Bocharov E V, et al., 2010)
Figure 1. Spatial structure of the EphA2tm dimer. (Bocharov E V, et al., 2010)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| EphA1 transmembrane segment dimer, pH 6.3 (expressed in E. coli) | Homo sapiens | Solution NMR | / | 2K1L |
| EphA2 transmembrane segment dimer (expressed in E. coli) | Homo sapiens | Solution NMR | / | 2K9Y |
Table 1. Structural Research of Erythropoietin-Producing Hepatocellular Receptors.
The NMR services provided by Creative Biostructure include but not limited in solution-state NMR, solid-state NMR, and paramagnetic NMR. Solution-state NMR is a powerful tool for determining the three-dimensional structure of biological molecules such as proteins and nucleic acids in solution. On the other hand, solid-state NMR is ideal for studying the structure and dynamics of molecules in their native solid-state environments. Our paramagnetic NMR service is especially useful for studying metal-containing proteins and other paramagnetic molecules. No matter what type of molecule you are working with, we have the expertise and technology to help you obtain the insights you need to move your research forward.
Our team of experienced scientists use state-of-the-art techniques to obtain high-quality NMR spectra and derive structural information from them. They also offer a range of other structural analysis techniques, such as X-ray crystallography and cryo-electron microscopy (cryo-EM), to provide a comprehensive understanding of the structure and function of biological molecules. If you are interested in our structural analysis services for Eph receptors and their ligands, please contact us to discuss your project. We are happy to provide a free consultation to help you determine the optimal approach for your research needs.
References
- Bocharov E V, et al. Spatial Structure and pH-dependent Conformational Diversity of Dimeric Transmembrane Domain of the Receptor Tyrosine Kinase EphA1. Journal of Biological Chemistry. 2008, 283(43): 29385-29395.
- Bocharov E V, et al. Left-handed dimer of EphA2 transmembrane domain: helix packing diversity among receptor tyrosine kinases. Biophysical Journal. 2010, 98(5): 881-889.