Cryo-Electron Microscopy (Cryo-EM) Services
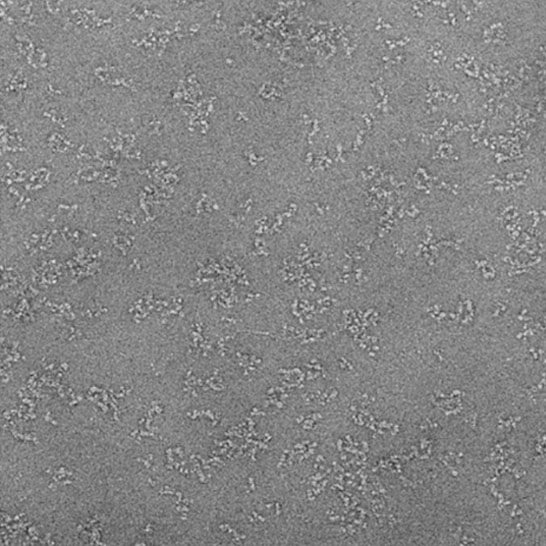
Cryo-electron microscopy (Cryo-EM) is a popular method for studying the ultrastructure of biological entities by rapidly freezing samples and irradiating them with electrons. Through electron scattering detection and computer reconstruction, sample structures can be determined. It's seen as an alternative to X-ray crystallography for exploring new targets. However, achieving high-resolution structures with Cryo-EM relies on obtaining high-quality protein samples and establishing necessary infrastructure for method feasibility assessment.
Why Do You Need This Service? (Using protein as an example)
- You have a small amount of materials.
- The material is hard to crystallize.
- You are looking into the molecules in atomic details.
- It is a large biomolecular complex (>150 kDa, up to 2000 Å).
- You want to observe the real native state structure.
Creative Biostructure's integrated cryo-EM platform covers all technical stages from gene synthesis to structure determination. You can utilize the full suite of recommended services or select individual service elements based on your project needs, such as protein production, feasibility analysis using negative staining TEM, and structure determination.
Gene-to-structure Services
- Construct design & gene synthesis: full-length, site-mutated, truncated, appropriate tag
- Protein expression : bacterial system, yeast system, insect cell/baculovirus system, mammalian cell system, plant system, cell-free protein production
- Protein purification : multiple chromatographic methods and rigorous analytics-driven quality control
Custom Cryo-EM Services
- Negative staining TEM feasibility analysis only
- Cryo-EM data collection only (using Glacios and/or Titan Krios)
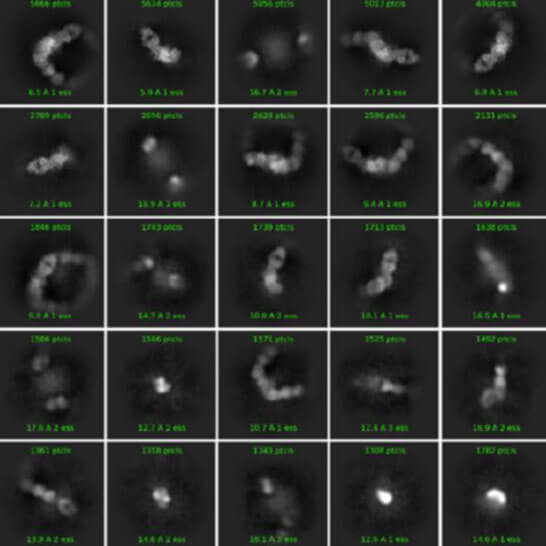
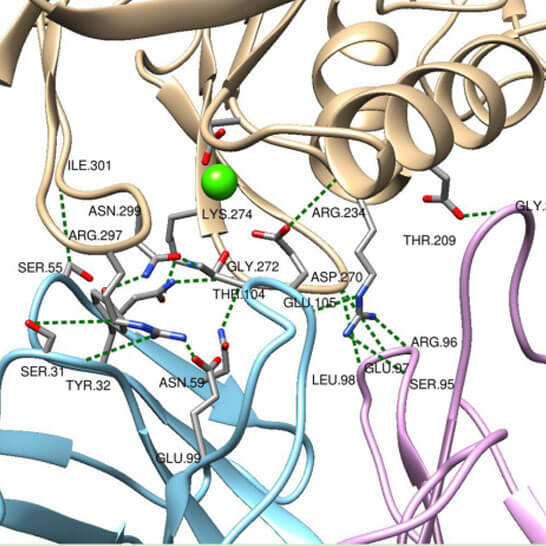
- Cryo-EM structure determination only (3D structure reconstruction, model building, refinement, and quality verification)
Our Featured Cryo-EM Services
Your Specific Sample Types Observed by Cryo-EM
Technology Platforms
This technology platform is equipped with highly specialized instruments for scanning electron microscopy (SEM), transmission electron microscopy (TEM), and the auxiliary equipment required for cryo-EM.
Electron Microscopy (EM) Platform
This technology platform relies on high-throughput screening technologies for the expression, purification, and structural characterization of difficult membrane proteins, such as GPCRs, ion channels, transporters, and GPI-anchored proteins.
Membrane Protein Platform
Key Advantages
- Comprehensive Protein Science Expertise
- - Engage with our dedicated team specializing in protein construct design, expression, and purification.
- - Benefit from meticulous quality control procedures ensuring the integrity and purity of your samples, optimized specifically for cryo-EM analysis.
- Advanced Cryo-EM Capabilities
- - Investigate the feasibility of cryo-EM, including negative staining TEM, for the evaluation of sample compatibility and the refinement of imaging parameters.
- - Leverage our expertise in cryo-EM condition optimization and high-resolution structure determination, empowering precise molecular insights into your targets.
- Target-Specific Proficiency
- - Access specialized knowledge tailored to diverse target classes, enhancing the efficacy and accuracy of structural elucidation across a wide range of biomolecules.
Resources
Frequently Asked Questions
-
How does cryo-EM compare to other structural biology techniques?
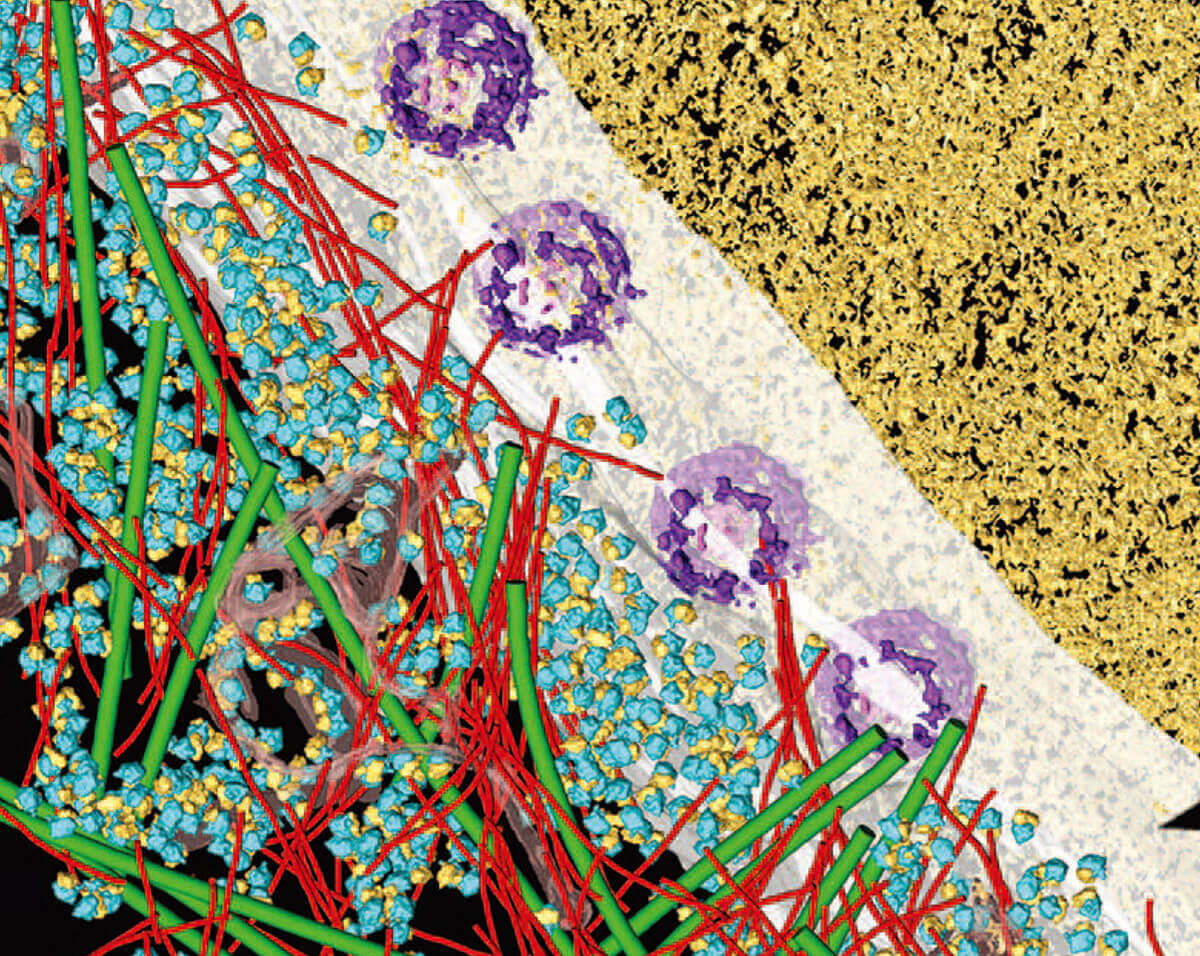
Cryo-electron microscopy has several advantages over other structural biology techniques, such as X-ray crystallography and nuclear magnetic resonance spectroscopy. Cryo-EM can visualize molecules in their native, biologically relevant state, without the need for crystallization or chemical modification. Cryo-EM can also image larger and more complex molecules than X-ray crystallography, making it particularly useful for studying viruses and large macromolecular complexes.
-
What is the resolution limit of cryo-EM?
The resolution limit of cryo-EM is determined by a combination of factors, including the quality of the electron microscope, the quality of the sample, and the data processing methods used. Currently, cryo-EM can achieve resolutions down to 1.2-1.5 Å, which is approaching the resolution of X-ray crystallography.
-
I want to use cryo-EM to determine protein structure, what are the requirements for sample preparation?
- Purification: The protein sample should be pure and free from contaminants to minimize the noise in the images and improve resolution.
- Concentration: The sample should be concentrated to a high enough concentration.
- Vitrification: The sample must be rapidly frozen to a temperature below its glass transition temperature, forming a solid but amorphous glass, in order to preserve the native state of the protein.
- Support: The sample should be placed on a thin film or grid to allow electrons to pass through and be captured by a detector.
- Stabilization: The sample may need to be stabilized with cryoprotectants or detergents to prevent aggregation or denaturation during the preparation and imaging process.
- These requirements should be optimized for each protein of interest to maximize the chances of obtaining high-resolution structures. At Creative Biostructure, you only need to provide the protein solution upon request, or tell us your target protein sequence, to achieve the research goal from sequence to structure.
Ordering Process
References
- Grassucci R A, et al. Preparation of macromolecular complexes for cryo-electron microscopy. Nature Protocols. 2007, 2(12): 3239.
- Doerr A. Single-particle cryo-electron microscopy. Nature Methods. 2016, 13(1): 23-23.
- Thompson R F, et al. An introduction to sample preparation and imaging by cryo-electron microscopy for structural biology. Methods. 2016, 100: 3-15.
- Mahamid J, et al. Visualizing the molecular sociology at the HeLa cell nuclear periphery. Science. 2016, 351(6276): 969-972.