Isothermal Titration Calorimetry (ITC)
Creative Biostructure provides highly sensitive and flexible MagHelix™ ITC service for studying binding affinity, stoichiometry, entropy, enthalpy and thermodynamics of biomolecular interactions. It works by directly measuring the heat that is either released or absorbed during a biomolecular binding event.
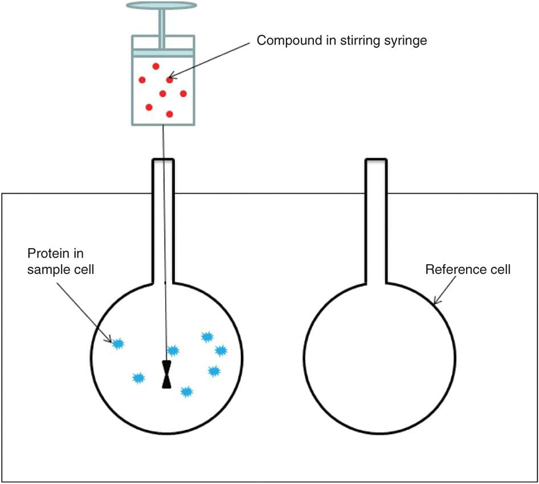
Figure. An ITC instrument consists of a stirring syringe, a sample cell and a reference cell.
Calorimetric based techniques, such as ITC, SPR, etc., have been useful tools for studying biomolecules interaction in biochemical researches. Isothermal Titration Calorimetry (ITC) is a useful quantitative method to measure the complete thermodynamic binding reaction and it is the only method that measures all binding parameters in a single experiment. There are two types of ITC instruments, single- and dual-injection variety. An ITC instrument consists of two lollipop-shaped identical cells which are made of a highly efficient thermal conducting material (hasteloy or gold) surrounded by an adiabatic jacket (shown in the figure). In an ITC experiment, the reference cell only contains buffer such as water and buffer with macromolecule added in the sample cell. Prior to the injection of the interested protein, the microcalorimeter helps to keep the two cells in the same temperature. After the protein of interest is inserted into the sample cell and if there is a binding between the sample and protein, heat changes can be detected. The heaters will have a feedback and then increase or decrease power to the sample cell to compensate for this difference to maintain the equal temperature. Typically, the protocols of an ITC experiment include sample preparation, loading the reference and sample cells, filling and attachment of the injection syringe, setting experimental parameters, control experiments to determine heats of dilution and data analysis. From an ITC experiment, the thermodynamic properties of biomolecules interaction can be measured directly and the values including the binding constant (Ka), the enthalpy of binding (ΔHb) and the stoichiometry (n) can also be determined. Therefore, ITC has been widely used in drug discovery and development. To quantify binding affinity, determine binding specificity and stoichiometry, measure enzyme kinetics or select candidates, ITC is an available quantitative means.
As a competitive bio-company in the world, Creative Biostructure provides ITC service to support your drug development and other researches.
Our MagHelix™ analytical methods for biophysical characterization of biomolecules include but are not limited to:
- Protein Thermal Shift Assay
- Surface Plasmon Resonance
- Isothermal Titration Calorimetry
- Differential Scanning Calorimetry
- Saturation-Transfer Difference
- Bio-Layer Interferometry (BLI) Technology
Xingding Zhou, et al.Application of isothermal titration calorimetry and column chromatography for identification of biomolecular targets.Nature Protocol, 2011 Feb; 6 (2):158-65.
Michael M. Pierce, et al. Isothermal titration calorimetry of protein-protein interaction. Methods. 1999 Oct;19(2):213-21.
Ordering Process
