MemPro™ Glucose transporter
Creative Biostructure provides custom MemPro™ gene-to-structure services ( from production to structure determination) for glucose transporter (GLUT).
The GLUT protein family belongs to the Major Facilitator Superfamily (MFS) of membrane transporters. To date, about 5,000 members of the MFS have been identified, encompassing all three kingdoms. Most GLUT proteins catalyze the bidirectional transfer of glucose across membranes, and they may exhibit either symmetric or asymmetric transport kinetics. GLUTs generally have approximately 500 amino acids and are predicted to possess 12 transmembrane-spanning alpha helices and a single N-linked oligosaccharide.
Class I GLUT are the most abundance and well-characterized glucose transporter in mammalian system. It can be further divided into 4 subclasses due to their distribution and functions.
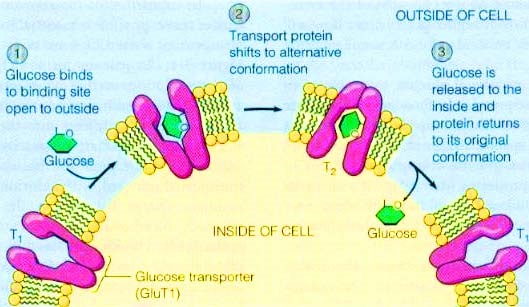 Figure 1. The alter conformation of GLUT to transport glucose across the membrane
Figure 1. The alter conformation of GLUT to transport glucose across the membrane
GLUT1 catalyzes the rate-limiting step in supplying cells of the central nervous system with glucose, an essential fuel for these cells. The ability to acutely upregulate GLUT1 activity might have positive protection to counter the effect of strokes due to arterial blockage as well as the damage that occurs to cardiomyocytes during cardiac infarction. Additionally, GLUT1 is frequently observed to have abnormal high expression level during oncogenesis in many different tissue types, a process that is probably crucial for tumors to grow beyond a size limited by their glycolytic capacity (the Warburg effect). The rational design of isoform-specific inhibitors of GLUT1 is of obvious interest in this regard.
GLUT2 has a uniquely high Km for glucose (∼17 mM), and it is expressed at a very high level in in the basolateral membranes of intestinal and kidney epithelial cells and pancreatic β-cells and of hepatocytes. Absence of GLUT2 prevents glucose-stimulated insulin secretion by β-cells and the regulation of glucose-sensitive gene expression in hepatocytes. Studies with gene knockout mice have indicated that GLUT2 is also required for the function of glucose sensors present in the hepatoportal vein area and in the central nervous system. These sensors appear to control glucagon secretion, insulin secretion, feeding behavior, and peripheral tissue glucose uptake
GLUT3 is the major neuronal glucose transporter, present in both dendrites and axons, and its level of expression in different regions of the brain correlates with regional cerebral glucose utilization (rCGU). GLUT3 has a high affinity for glucose (Km ∼1.5 mM) to ensure efficient glucose uptake by neuron due to its highest calculated turnover number of the GLUT isoforms. Knockout of GLUT3 in the mouse induces apoptosis in the embryos. GLUT3 is also expressed in immune cells including lymphocytes, monocytes/macrophages, and platelets. In these cells, it is present in intracellular vesicles that can translocate and fuse to the plasma membrane upon cellular activation to ensure increased glucose uptake and metabolism.
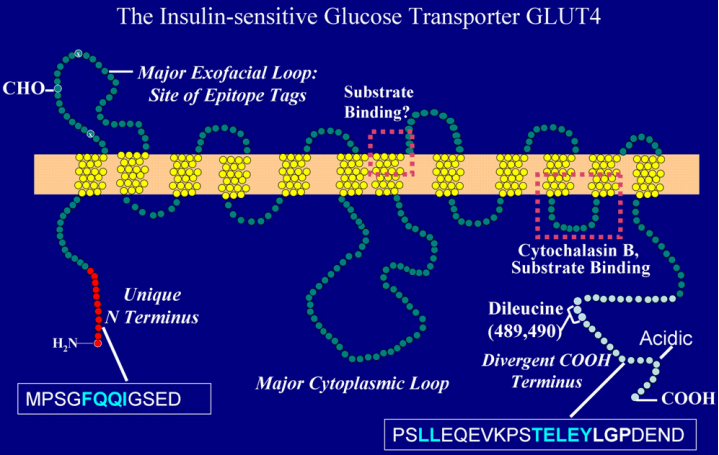 Figure 2. The Structure of GLUT4
Figure 2. The Structure of GLUT4
GLUT4 has elusive mechanism of regulation by insulin and the disruption of this regulation in several prevalent insulin-resistant states, including obesity and type 2 diabetes mellitus. Insulin induce GLUT4 translocation from intracellular membrane compartments to the cell surface in adipocytes and skeletal muscle. Overexpression of GLUT4 expression in skeletal muscle shows insulin sensitivity and glucose tolerance. Conversely, conditional depletion of GLUT4 in either adipose tissue or skeletal muscle causes insulin resistance and a roughly equivalent incidence of diabetic.
References:
Deng D, Sun P, Yan C, et al. Molecular basis of ligand recognition and transport by glucose transporters[J]. Nature, 2015.
Thorens B, Mueckler M. Glucose transporters in the 21st Century[J]. American Journal of Physiology-Endocrinology and Metabolism, 2010, 298(2): E141-E145.
