Structural Research of Adenylyl Cyclases
Membrane-integral adenylyl cyclases (ACs) are crucial proteins in mammalian signal transduction, responding to a large number of intracellular and extracellular signals, such as GTP-binding protein (G protein)-dependent signaling, lipid signaling cascades, and changes in intracellular Ca2+ concentration, and generating cyclic adenosine monophosphate (cAMP) for a series of downstream signaling events.
Mammalian ACs have nine subtypes from AC1 to AC9. Based on the amino acid sequence, all AC subtypes share the same overall predicted structure. They are membrane proteins composed of 12 transmembrane (TM) helices with cytosolic N and C termini. The first six TM domains (TM1-TM6) of the AC are followed by a cytosolic region containing the catalytic domain (C1a), then the C1b domain, the second TM region, the TM7-TM12 bundle, and then the second catalytic domain (C2a) and C-terminal C2b domain. However, each AC subtype contains additional structural elements, such as the helical domain (HD) and the membrane-spanning domain, which are significant for the proper assembly of these proteins and their enzymatic function.
A deep understanding of the role of AC in signal transduction has long been lacking because of the challenges of expressing and purifying ACs for structural studies. A recent study reported the cryo–electron microscopy (cryo-EM) structure of bovine membrane AC9 (3.40 Å) and determined the structure of bovine AC9 in complex with Gas utilizing cryo-EM and single-particle analysis. AC9 is an AC subtype expressed in many tissues, such as heart, brain and lung. The structure reveals the organization of membrane domains and helical domains spanning between the membrane and catalytic domains of AC9. The carboxy-terminal extension of the catalytic domain blocks the catalytic and allosteric sites of AC9, inducing a conformation distinct from the activator- and substrate-bound state, suggesting a regulatory role in cAMP generation.
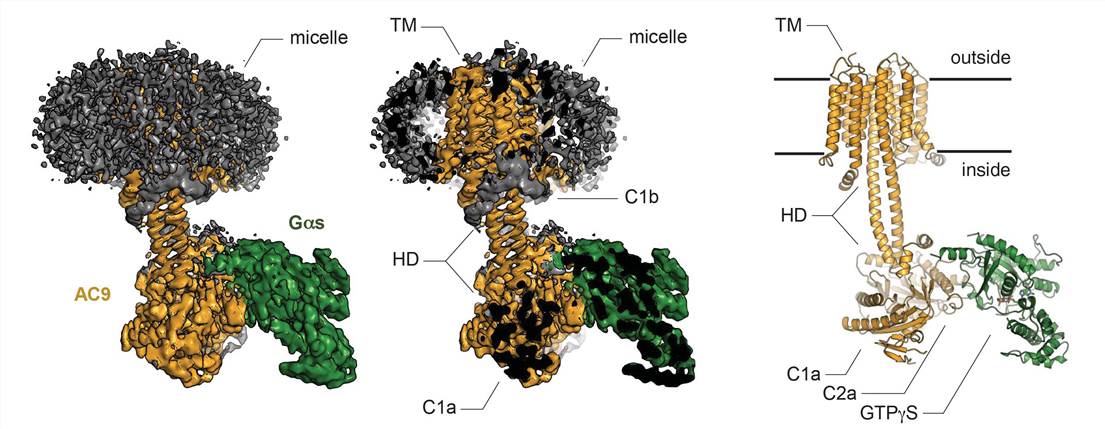 Figure 1. Cryo-EM structure of the AC9-Gas complex. (Qi C, et al., 2019)
Figure 1. Cryo-EM structure of the AC9-Gas complex. (Qi C, et al., 2019)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Adenylyl cyclase AC9 bound to an activated stimulatory G protein (expressed in Trichoplusia ni) | Bos taurus | Cryo-EM single particle analysis | 3.40 Å | 6R3Q |
| Truncated adenylyl cyclase bound to MANT-GTP, forskolin and an activated stimulatory Galphas protein (expressed in Trichoplusia ni) | Bos taurus | Cryo-EM single particle analysis | 4.20 Å | 6R4O |
| Soluble domain of adenylyl cyclase bound to an activated stimulatory G protein (expressed in Trichoplusia ni) | Bos taurus | Cryo-EM single particle analysis | 3.10 Å | 6R4P |
Table 1. Structural Research of Adenylyl Cyclases.
In recent years, the structural analysis of biomolecules using cryo-EM has become a crucial research direction in structural biology, especially for the study of membrane protein structures. At the same time, the obtained cryo-EM structures deepen the understanding of biomolecular functions, their roles in diseases, and targeted drug development.
Creative Biostructure has been committed to the research of structural biology and membrane protein for a long time and has rich experience in using single-particle analysis to determine the structure of membrane protein. In addition to the structural analysis of membrane proteins, we can accurately analyze other biomolecules including but not limited to nucleic acids, small proteins, ribosomes, protein complexes, protein-ligand complexes, and viruses. If you are interested in our services, please feel free to contact us.
Reference
- Qi C, et al. The structure of a membrane adenylyl cyclase bound to an activated stimulatory G protein. Science. 2019. 364(6438): 389-394.