Structural Research of Arenaviridae
The Arenaviridae family can be divided into two types according to genotype, serotype, and appearance area, including the Old World complex (such as Lassa fever virus, lymphocytic choriomeningitis virus) and the New World complex (such as Tacaribe virus, Junin virus and Machupo virus). The virions of this family are spherical, about 50-300 nm in diameter, have a dense lipid envelope, and have a surface layer covered by club-like protrusions with 8-10 nm in length. The viral genome consists of two single-stranded, ambiguous RNA molecules, L (1arge) chain and S (small) chain, approximately 7.5 kb and 3.5 kb in length, respectively. The most abundant structural proteins are nucleoproteins (NP), and non-ribosylated polypeptides (about 63kDa), which are found to be closely related to viral genomic RNA in the form of ribonucleoprotein complexes or nucleocapsid structures. Lipids comprise about 20% of the dry weight of virions and are similar in composition to those of the host plasma membrane. The carbohydrates in the form of complex glycans on glycoproteins including GP1 and GP2 account for about 8% of the dry weight of the virions. The infection process involves attachment to cellular receptors, entry through the endosomal pathway, and transcription of mRNA in the cytoplasm of uncoated and infected cells. The viral envelope glycoprotein is synthesized in cells as a single mannose-rich precursor molecule that is proteolytically cleaved and processed to contain complex glycans during transport to the plasma membrane.
Lassa fever virus
Lassa fever is an acute viral disease caused by the Lassa fever virus. Although the disease was first discovered in the 1950s, it was not until 1969 that the virus was isolated. Its clinical manifestation varies widely, may be the asymptomatic infection, also may be fatal to come on. Lassa fever causes considerable morbidity and mortality in West Africa. Around the world, Lassa fever has been a focus of public health officials. It is estimated that more than 200,000 new infections occur each year in West Africa, killing more than 3,000 people. The mortality rate of Lassa fever in hospitalized patients is about 15%, and in several outbreaks, the mortality rate is over 50%. Contact with Mastomys natalensis (a widely distributed, highly symbiotic rodent) and patients with Lassa fever are the main routes of Lassa fever virus infection. In addition, the Lassa fever virus is also listed as a Class A biological weapon preparation.
The Lassa fever virus genome consists of two ambisense single-stranded RNAs, a 3.4 kb S (small) chain and a 7.2 kb L (1arge) chain. The two genes of the S chain encode three structural proteins: nucleoproteins (NP), envelope glycoprotein GP1 and GP2. The two genes of the L chain encode a viral polymerase (L protein) and a matrix protein (Z). NP and L proteins bind to RNA to form a ribonueleoprotein or participate in the formation of a nucleocapsid. The Z protein is generally considered to be involved in the formation of viral particles as a matrix protein. GP1 and GP2 are cleaved by their precursor glycoprotein C (GPC) via the cellular protease SKI-1/S1P. GP1 is a part of the surface glycoprotein spike that acts as a receptor-binding site. GP2 has a consistent structure with other enveloped viral transmembrane fusion proteins.
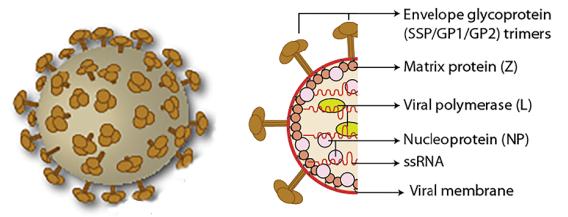 Figure 1. Arenavirus virion structure.
Figure 1. Arenavirus virion structure.
Highly purified virus-like particles (VLPs) can be used to study the structural properties of natural viruses. They are a safe option for drug R&D since they can trigger powerful immune responses and do not contain viral genetic material. Creative Biostructure offers off-the-shelf VLP products that are ready for immediate worldwide delivery. Moreover, you can have easy access to cryo-electron microscopy and greatly accelerate your research on the structural biology of viruses.
Reference
- Burri D J, et al. Envelope Glycoprotein of Arenaviruses. Viruses. 2012. 4(10): 2162-2181.