Structural Research of G Protein-coupled Receptors (GPCRs) Class D
G protein-coupled receptors (GPCRs) are a superfamily of membrane proteins that play crucial roles in cellular signaling. They are involved in diverse physiological processes and serve as key targets for many pharmaceutical drugs. The GPCRs are phylogenetically divided into six classes, namely A, B, C, D, E, and F. While substantial progress has been made in understanding the structures and functions of GPCRs from classes A, B, C, and F, Class D GPCRs, found exclusively in fungi, have remained relatively elusive.
A recent study has successfully determined the first structure of a class D GPCR, the Saccharomyces cerevisiae pheromone receptor Ste2, in an active state coupled to the heterotrimeric G protein Gpa1-Ste4-Ste18. Ste2 functions as a homodimer, with the dimer interface formed by specific regions, such as the N-terminus, transmembrane helices H1, H2, H7, and the first extracellular loop ECL1. The structure of Ste2 exhibits similarities in overall topology to class A GPCRs; however, it possesses unique features, such as a shift of helix H4 by over 20 Å and a shallow groove as the G protein binding site, as opposed to the typical cleft found in class A GPCRs.
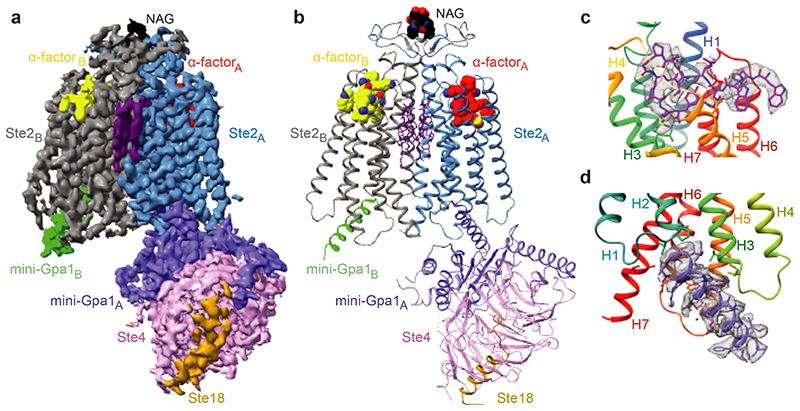 Figure 1. Overall cryo-EM reconstruction of the Ste2-G protein heterotrimer complex. (Velazhahan V, et al., 2021)
Figure 1. Overall cryo-EM reconstruction of the Ste2-G protein heterotrimer complex. (Velazhahan V, et al., 2021)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Class D GPCR Ste2 dimer coupled to two G proteins (expressed in Trichoplusia ni) | Saccharomyces cerevisiae | Cryo-EM single particle analysis | 3.30 Å | 7AD3 |
| ligand-free GPCR dimer Ste2 (expressed in Trichoplusia ni) | Saccharomyces cerevisiae | Cryo-EM single particle analysis | 3.10 Å | 7QB9 |
Table 1. Structural research of class D GPCRs.
Creative Biostructure's expertise in structural biology, particularly in GPCRs research, positions us as a reliable partner for academic and pharmaceutical institutions seeking comprehensive structural analysis services. Our advanced cryo-electron microscopy (cryo-EM) techniques and cutting-edge technologies enable high-resolution imaging and accurate determination of complex structures like Ste2 in its active state.
Our highly skilled team of scientists holds extensive expertise in GPCR biology and leverages cutting-edge computational tools to analyze and interpret intricate structural data. Our unwavering dedication lies in delivering top-tier services and fostering collaborations with clients to make significant advancements in the comprehension of class D GPCR. Contact us and explore how our state-of-the-art capabilities can empower your research, propelling you closer to accomplishing your scientific aspirations.
References
- Velazhahan V, et al. Structure of the class D GPCR Ste2 dimer coupled to two G proteins. Nature. 2021, 589(7840): 148-153.
- Velazhahan V, et al. Activation mechanism of the class D fungal GPCR dimer Ste2. Nature. 2022, 603(7902): 743-748.