Structural Research of Type VII Secretion Systems
The type VII secretion system (T7SS) plays a critical role in the secretion of effector proteins from mycobacteria and is central to pathogen virulence. Mycobacteria that cause tuberculosis survive in the host and effectively evade the immune response through the homologous T7SS. Because of the essential importance of T7SS for mycobacterial physiology and virulence, T7SSs are considered promising targets for the development of therapeutic or prophylactic drugs against tuberculosis. Therefore, research on the structure and protein transport mechanisms of T7SS provides opportunities to develop new therapies against related diseases.
Basic structural analysis of T7SS
T7SS are complex protein nanomachines embedded in the mycobacteria cell envelope. In pathogenic bacteria, a total of five different T7SSs (ESX-1 to ESX-5) are the main secretion pathway for about 200 proteins. The differential regulation between the T7SSs contributes to the secretion of substrates during infection. To date, seven conserved genes are required for the secretion process and are considered to be core components of the T7SS. Among them, two core components (EspG and EccA) are localized in the Mycobacterium cytosol, while the other five (EccB, EccC, EccD, EccE, and MycP) contain transmembrane structural domains (TMDs) and reside in the envelope.
Progress in research on the overall structure of the T7SS
In recent years, researchers have reconstructed the ESX-5 T7SS of Mycobacterium tuberculosis H37Rv to obtain a structural view of the entire T7SS membrane complex of this human pathogen. Cryo-electron microscopy (cryo-EM) analysis revealed ESX-5 as a well-defined hexameric structure comprising EccB5, EccC5, EccD5, EccE5, and MycP5, anchored to the inner membrane by 165 transmembrane helices (TMH). The membrane assemblies were described as trimers of dimers, where each dimer contained one MycP5 and two protomers, and each protomer contained EccB5, EccC5, EccE5, and two EccD5. The periplasm of ESX-5 consists of three EccB5 dimers and three MycP5 proteases. The EccB5 dimers are assembled in a triangular shape forming a central cavity.
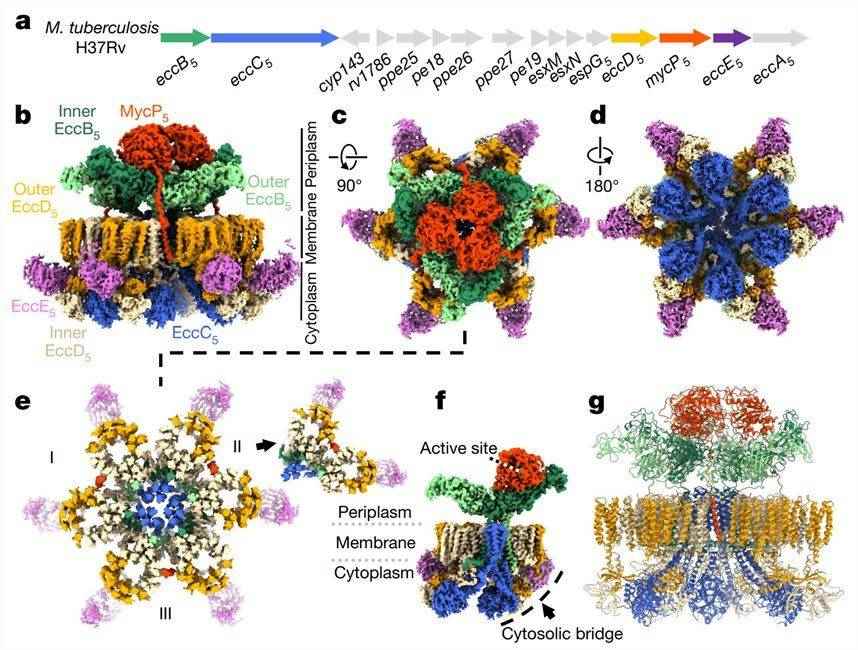 Figure 1. Cryo-EM structure of the intact ESX-5 inner-membrane complex of M. tuberculosis. (Bunduc CM, et al., 2021)
Figure 1. Cryo-EM structure of the intact ESX-5 inner-membrane complex of M. tuberculosis. (Bunduc CM, et al., 2021)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| ESX-5 Type VII Secretion System hexameric pore complex | Mycobacterium xenopi RIVM700367 | Cryo-EM single particle analysis | 3 Å | 7B9F |
| ESX-5 Type VII Secretion System hexameric pore complex | Mycobacterium xenopi RIVM700367 | Cryo-EM single particle analysis | 3.4 Å | 7B9S |
| EccB1 in spacegroup P21 (state III) | Mycobacterium tuberculosis H37Rv | X-ray diffraction | 3 Å | 5EBC |
| EccB1 in spacegroup P21 (state IV) | Mycobacterium tuberculosis H37Rv | X-ray diffraction | 2.6 Å | 5EBD |
| ESX-3 complex | Mycolicibacterium smegmatis MC2 155 | Cryo-EM single particle analysis | 3.7 Å | 6LAR |
| ESX-3 core complex | Mycolicibacterium smegmatis MC2 155 | Cryo-EM single particle analysis | 3.8 Å | 6SGW |
| ESX-3 translocon complex | Mycolicibacterium smegmatis MC2 155 | Cryo-EM single particle analysis | 3.7 Å | 6UMM |
| Protomer 1 of the ESX-3 core complex | Mycolicibacterium smegmatis MC2 155 | Cryo-EM single particle analysis | 3.7 Å | 6SGX |
| EccB3 dimer from the ESX-3 core complex | Mycolicibacterium smegmatis MC2 155 | Cryo-EM single particle analysis | 4.6 Å | 6SGY |
| Protomer 2 of the ESX-3 core complex | Mycolicibacterium smegmatis MC2 155 | Cryo-EM single particle analysis | 3.9 Å | 6SGZ |
| Cytosolic bridge of an intact ESX-5 inner membrane complex | Mycobacterium tuberculosis H37Rv | Cryo-EM single particle analysis | 3.27 Å | 7NPT |
| MycP5-free ESX-5 inner membrane complex, state I | Mycobacterium tuberculosis H37Rv | Cryo-EM single particle analysis | 4.48 Å | 7NPU |
| MycP5-free ESX-5 inner membrane complex, State II | Mycobacterium tuberculosis H37Rv | Cryo-EM single particle analysis | 6.66 Å | 7NPV |
| Intact ESX-5 inner membrane complex, Composite C1 model | Mycobacterium tuberculosis H37Rv | Cryo-EM single particle analysis | 4.03 Å | 7NP7 |
| Intact ESX-5 inner membrane complex, Composite C3 model | Mycobacterium tuberculosis H37Rv | Cryo-EM single particle analysis | 3.82 Å | 7NPR |
| N-terminal domain of EccA1 ATPase from ESX-1 secretion system | Mycobacterium tuberculosis | X-ray diffraction | 2 Å | 4F3V |
| The TPR-rich domain of EccA3 | Mycolicibacterium smegmatis MC2 155 | X-ray diffraction | 1.6 Å | 7NAZ |
| The cytoplasmic domain of EssC | Geobacillus thermodenitrificans | X-ray diffraction | 2.91 Å | 5FVO |
Table 1. Structural research of the type VII secretion systems.
Creative Biostructure is a leader in the field of biomolecular structural research, offering personalized solutions and flexible collaboration opportunities to satisfy clients' specific requirements. Whether it is basic structural research or analysis of protein secretion function in type VII secretion systems, we provide accurate and reliable results.
With cutting-edge technologies such as X-ray crystallography, cryo-electron microscopy (cryo-EM), and NMR spectroscopy, we can provide high-resolution structural analysis services. Our team consists of experienced scientists and researchers dedicated to solving intricate biological problems. If you need biomolecular structural research, contact us for more details. Our services will help you get closer to achieving your scientific goals.
References
- Bunduc CM, et al. Structure and dynamics of a mycobacterial type VII secretion system. Nature. 2021. 593(7859): 445-448.
- Bunduc CM, et al. Structure and Function of the Mycobacterial Type VII Secretion Systems. Annu Rev Microbiol. 2020. 74: 315-335.
- Famelis N, et al. Mycobacterial type VII secretion systems. Biol Chem. 2023. 404(7): 691-702.