Structural Research of PIN-FORMED (PIN) Auxin Efflux Carriers
Auxins is a hormone with a central role in controlling almost all plant growth and development. Research has found that FORMED (PIN) family members control the export of Auxins from the cytoplasm to the extracellular space. Researchers found that all PIN proteins have conserved structures at resolutions of 2.9 to 3.4 Å using cryo-electron microscopy (Cryo-EM). These structures indicate the molecular mechanisms and models of auxin transport, which are significant for explaining the central mechanism of polar auxin transport.
Functions of PIN Proteins in Plants
PIN proteins perform unique functions in auxin signaling-mediated development and growth. These include embryonic development, lateral root development, shoot vascular development, flower bud formation, and abiotic stress responses. For example, AtPIN1 in Arabidopsis thaliana is mainly involved in the maintenance of the embryonic Auxins gradient, and AtPIN2 is associated with basal leaf transport and geotropism. In Oryza sativa, OsPIN1b functions in auxins-dependent adventitious root emergence and tillering. OsPIN3t is implicated in drought stress response and drought tolerance. In Zea mays, ZmPIN1a and ZmPIN1b are involved in endosperm and embryo development.
Advances in PIN protein structure research
Researchers analyzed the symmetric dimer conformation of Arabidopsis thaliana apo PIN8 using the single-particle cryoelectronic microscope. It has a double rotational axis perpendicular to the membrane plane, extending from the non-cytoplasmic side into the membrane. Each monomer contains ten transmembrane helices (M1-M10). apo-PIN8 monomer is divided into two structural domains, the scaffold domain and the transporter protein domain. The scaffolding domain contains M1, M2, M6, and M7, and is mediated mainly by M2 and M7 and further stabilized by lipids between M1 and M6. The transporter protein domain consists of M3-M5 and M8-M10 and contains a central X-shaped cross. Next to the crosses, water-filled binding pockets open up to the non-cytoplasmic side of the protein through the concave surface.
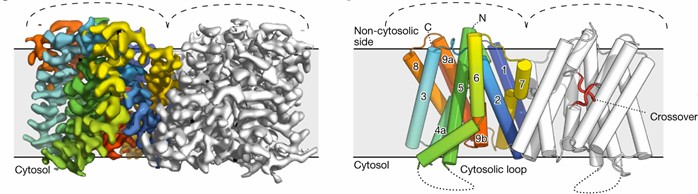 Figure 1. Schematic structure of the PIN8 dimer. (Ung KL, et al., 2022)
Figure 1. Schematic structure of the PIN8 dimer. (Ung KL, et al., 2022)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Outward-facing apo-form of auxin transporter PIN8 | Arabidopsis thaliana | Cryo-EM single particle analysis | 2.89 Å | 7QP9 |
| Outward-facing auxin bound form of auxin transporter PIN8 | Arabidopsis thaliana | Cryo-EM single particle analysis | 3.18 Å | 7QPA |
| Inward-facing NPA bound form of auxin transporter PIN8 | Arabidopsis thaliana | Cryo-EM single particle analysis | 3.44 Å | 7QPC |
| Apo state of AtPIN3 | Arabidopsis thaliana | Cryo-EM single particle analysis | 3 Å | 7WKS |
| NPA bound state of AtPIN3 | Arabidopsis thaliana | Cryo-EM single particle analysis | 2.62 Å | 7WKW |
| IAA bound state of AtPIN3 | Arabidopsis thaliana | Cryo-EM single particle analysis | 2.93 Å | 7XXB |
| Auxin exporter PIN1 in the apo state | Arabidopsis thaliana | Cryo-EM single particle analysis | 3.1 Å | 7Y9T |
| Auxin exporter PIN1 in the NPA-bound state | Arabidopsis thaliana | Cryo-EM single particle analysis | 3.3 Å | 7Y9U |
| Auxin exporter PIN1 in the IAA-bound state | Arabidopsis thaliana | Cryo-EM single particle analysis | 3.2 Å | 7Y9V |
Table 1. Structural research of PIN-FORMED (PIN) auxin efflux carriers.
Structural analysis is essential for research on the function and action mechanisms of proteins and other biomolecules. Creative Biostructure is a leading provider of such services. Our team of experts uses various cutting-edge technologies to provide clients with high-quality structural information.
Cryo-electron microscopy (cryo-EM) is a powerful biology technique for researching the structure of PIN-FORMED (PIN) auxin efflux carriers. We provide one-stop cryo-EM services including sample preparation, data acquisition, image processing, and structure determination. If you have requirements in structure analysis of biological macromolecules, please feel free to contact us. We have the professional knowledge and abundant resources to deliver results exceeding your expectations.
References
- Ung KL, et al. Structures and mechanism of the plant PIN-FORMED auxin transporter. Nature. 2022.609(7927):605-610.
- Zhou JJ, Luo J. The PIN-FORMED Auxin Efflux Carriers in Plants. Int J Mol Sci. 2018.19(9):2759.
- Zwiewka M, et al. The Nuts and Bolts of PIN Auxin Efflux Carriers. Front Plant Sci. 2019.10:985.
- Qi L, et al. Characterization of the Auxin Efflux Transporter PIN Proteins in Pear. Plants (Basel). 2020.9(3):349.