Affinity Chromatography
As the worldwide leading suppliers of custom protein services, Creative Biostructure is pleased to provide free protein purification technical resources to facilitate your daily research based on our advanced MagHelix™ Protein Purification services. Affinity chromatography is involved with more than 60% of all purification techniques as stated, and affinity chromatography basically depends on a molecule bound to a column to recognize the target molecule.
There are three main steps in affinity chromatography purification:

- Binding of the target molecule in a crude sample to the immobilized ligand;
- Washing away other non-bounded components in the sample;
- Eluting of the target molecule by altering the buffer conditions, making the target molecule no longer bind to the immobilized ligand.
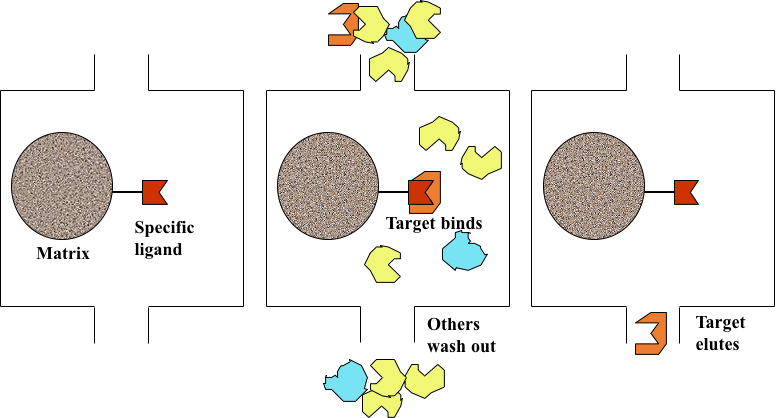 Figure 1. Illustration of three main steps in affinity chromatography
Figure 1. Illustration of three main steps in affinity chromatography
The ligand that is used for purification of its respective binding partner can be any component. There are some common biological interactions can be frequently applied in affinity chromatography, as follows:
- Antibody for antigen or virus;
- Enzyme for substrate analogue, inhibitor, or cofactor;
- Lectin for glycoprotein or cell surface receptor;
- Hormone/vitamin for receptor or carrier protein;
- Glutathione for glutathione-S-transferase or GST fusion proteins;
- Nucleic acid for histones, nucleic acid polymerase, or nucleic acid binding protein;
- Metal ions for native proteins with histidine, or cysteine and/or tryptophan residues.
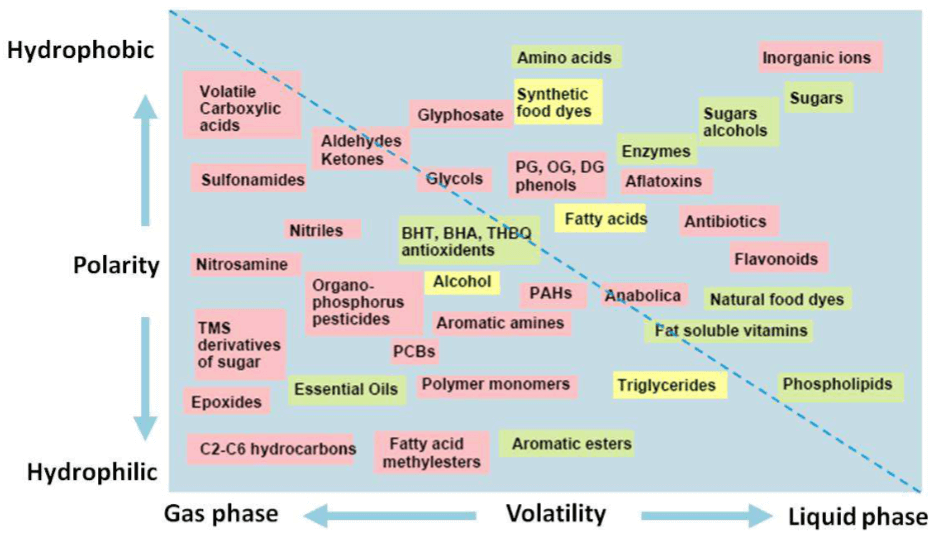 Figure 2. Illustration of a variety of molecules that can be purified by using affinity chromatography. (Affinity Chromatography Principles and Methods, 18-1022-29)
Figure 2. Illustration of a variety of molecules that can be purified by using affinity chromatography. (Affinity Chromatography Principles and Methods, 18-1022-29)
Affinity chromatography in practice
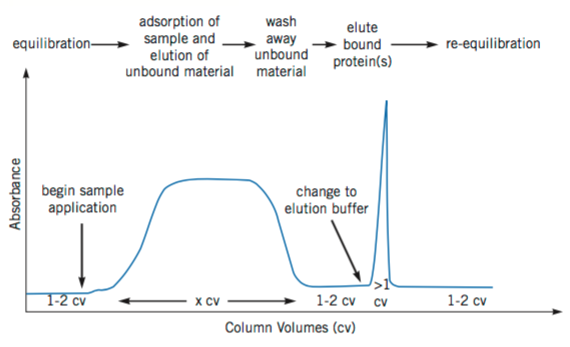 Figure 3. Illustration of a typical affinity purification. (Affinity Chromatography Principles and Methods, 18-1022-29)
Figure 3. Illustration of a typical affinity purification. (Affinity Chromatography Principles and Methods, 18-1022-29)
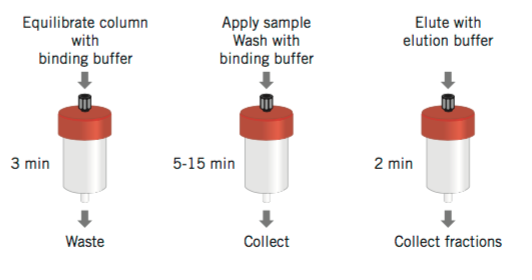 Figure 4. Illustration of simple procedures to perform affinity purification on a pre-packed HiTrap column. (Affinity Chromatography Principles and Methods, 18-1022-29)
Figure 4. Illustration of simple procedures to perform affinity purification on a pre-packed HiTrap column. (Affinity Chromatography Principles and Methods, 18-1022-29)
Things to Consider:
- Media selection (choose pre-packed columns, convenient and saving time);
- Media and buffer preparation (using high quality water and solutes, filtration is important);
- Sample preparation (binding affinity, removing components that interfere the binding, proper washing before elution);
- Elution (pH elution, ionic strength elution, competitive elution, reduced polarity of eluent, chaotropic eluents, elution gradient, flow rates)
Troubleshooting:
(Data were taken from Affinity Chromatography Principles and Methods, 18-1022-29)
1. Elution with a sharp target peak--Satisfactory result

2. Elution with a broad, low target peak while appling a binding buffer
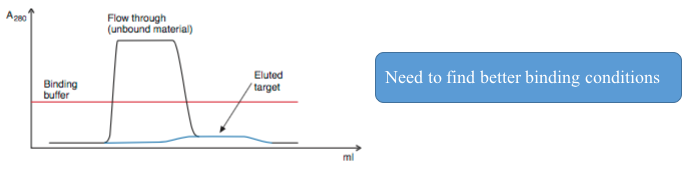
3. Elution with a broad, low target peak
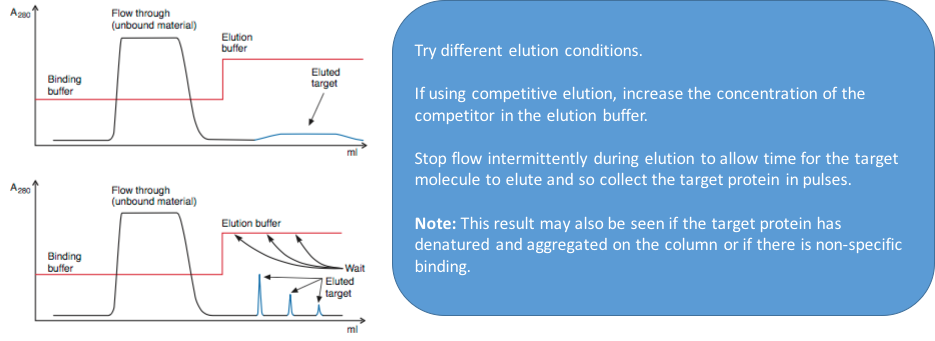
4. Elution with a broad, low target peak while still under binding conditions
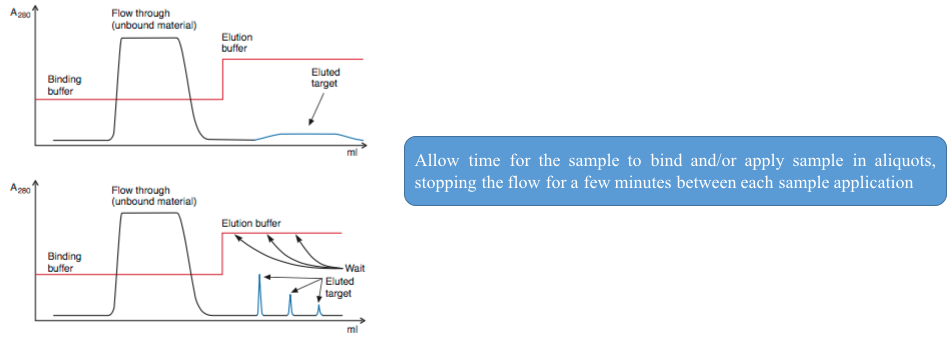
Our support team is currently establishing Support Centers for Protein Purification service, please see other technical resources as below and feel free to Contact Us.