Structural Research of Type IX Secretion System Motors
The type IX secretion system (T9SS) is a protein export system unique to the Bacteroidetes phylum and essential for the gliding of motile bacteria. Research has found that the T9SS consists of at least 21 protein components, of which only the PorL and PorM proteins share features with recognized PMF-dependent motors. Researchers have recently resolved the structure of PorL and PorM and facilitated advances in the overall interactome, providing new insights into the bacterial secretion mechanism.
Structural analysis of PorL and PorM
The natural structures of PorL and PorM were analyzed using cryo-electron microscopy. The structures show that PorL (GldL) and PorM (GldM) are located in the inner membrane of the T9SS. PorL has two transmembrane helices and a cytoplasmic structural domain, while PorM has one transmembrane helix and a periplasmic region, which interact with each other through transmembrane segments. The periplasmic region of PorM and PorM exists in a dimeric form and consists of four structural domains, D1 to D4, which can span most of the periplasmic space. D1 to D4 of PorM share the same straight topology, whereas in PorM there is a bend of about 45° between domains D2 and D3.
Structure and action mechanism of the PorLM motility complex
Researchers have resolved the core structure of the PorLM motor complex from the sliding bacterium Flavobacterium johnsoniae. The structure shows that the motor of T9SS is a PorL5PorM2 heteroheptamer, in which 10 TMHs of PorL surround two TMHs of PorM. Amino acid substitutions suggest that several conserved residues in the PorL and PorM transmembrane helices may be involved in coupling the proton flow across the inner membrane to the mechanical motion in the motor. Based on the structure of the complex, the researchers propose that PorLM forms a rotating motor in which the PorM subunit rotates within the loop of the PorL helix. The periplasmic structural domain of PorM transmits this rotational motion across the periplasm to the outer membrane components of the T9SS and the gliding motor system.
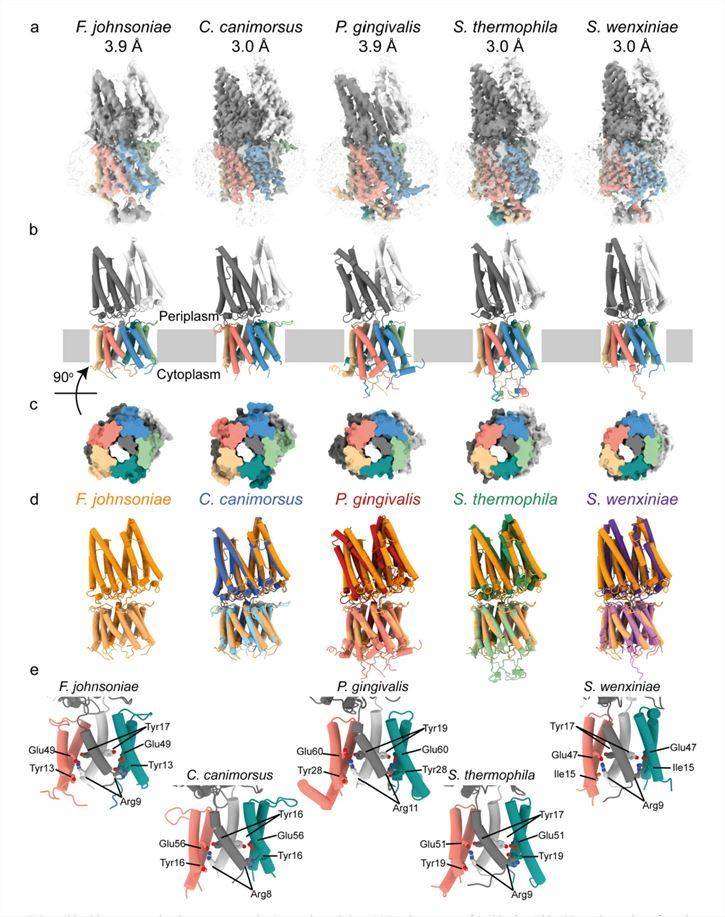 Figure 1. PorLM' has conserved architecture across the Bacteroidetes phylum. (Hennell James R, et al., 2022)
Figure 1. PorLM' has conserved architecture across the Bacteroidetes phylum. (Hennell James R, et al., 2022)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| GldLM | Flavobacterium johnsoniae | Cryo-EM single particle analysis | 3.9 Å | 6YS8 |
| GldLM | Schleiferia thermophila str. Yellowstone | Cryo-EM single particle analysis | 3 Å | 7SAU |
| GldLM | Sphingobacterium wenxiniae | Cryo-EM single particle analysis | 3 Å | 7SAX |
| GldLM | Capnocytophaga canimorsus Cc5 | Cryo-EM single particle analysis | 3 Å | 7SAZ |
| GldLM | Porphyromonas gingivalis ATCC 33277 | Cryo-EM single particle analysis | 3.9 Å | 7SAT |
| Periplasmic domain (residues 36-513) of GldM | Flavobacterium johnsoniae | X-ray diffraction | 2 Å | 6EY4 |
| C-terminal part (residues 224-515) of PorM | Porphyromonas gingivalis | X-ray diffraction | 2.85 Å | 6EY5 |
| C-terminal part (residues 315-516) of PorM with the nanobody nb130 | Porphyromonas gingivalis | X-ray diffraction | 2.1 Å | 6EY6 |
| N-terminal part (residues 30-212) of PorM with the nanobody nb01 | Porphyromonas gingivalis | X-ray diffraction | 2.4 Å | 6EY0 |
| Periplasmic domain of PorM | Porphyromonas gingivalis ATCC 33277 | X-ray diffraction | 3.7 Å | 7CMG |
| Periplasmic domain of GldM | Capnocytophaga canimorsus Cc5 | Cryo-EM single particle analysis | 3.4 Å | 7SB2 |
Table 1. Structural research of the type IX secretion system motors.
Creative Biostructure provides structural analysis services to help researchers explore the structure of type IX secretion systems motors. We offer a one-stop shop for protein expression, purification, and crystallization, as well as X-ray crystallography, cryo-electron microscopy (cryo-EM), and NMR spectroscopy for structure determination. Our team has many years of experience in the field of structural biology and can provide high-quality services tailored to specific research requirements.
If you are interested in the structure of membrane proteins or other biomolecules and would like to delve deeper into the science behind them, our team is at your service. We encourage you to contact us today to learn more about our comprehensive services and how we can help you achieve your goals.
References
- Hennell James R, et al. Structures of the Type IX Secretion/Gliding Motility Motor from across the Phylum Bacteroidetes. mBio. 2022. 13(3): e0026722.
- Gorasia DG, et al. The Type IX Secretion System: Advances in Structure, Function and Organization. Microorganisms. 2020. 8(8): 1173.
- Veith PD, et al. The Type IX Secretion System and Its Role in Bacterial Function and Pathogenesis. J Dent Res. 2022. 101(4): 374-383.