Structural Research of Fluc Family Fluoride Channels
The Fluc family of fluoride channels protects microorganisms from environmental fluoride by disrupting toxic halide accumulation. These proteins are structurally specific, and their permeation pathways and mechanisms have no parallels in other known ion channels. These channels are unusual in that they can assemble into symmetric homodimers with the two subunits arranged in an antiparallel transmembrane topology. This makes the intracellular and extracellular ion entry channels structurally identical.
These fluoride channel family molecule channels are also a class of protein channels widely found in bacteria and fungi. They play a significant role in regulating intra- and extracellular fluoride ion homeostasis. These channel proteins specifically transport fluoride ions, helping bacteria adapt and survive in environments containing high fluoride ion concentrations.
Crystallographic studies reveal a cation bound at the center of the protein, at the dimer interface. This cation coordinates with the main chain carbonyl oxygen atom from the membrane break in the two corresponding transmembrane helices. The protein has two deep-water vestibules with positive electrical properties due to an absolutely conserved arginine side chain and a deeply buried sodium ion in the center of the protein. These structures capture the electron density of four fluoride ions, two in each pore. They are located near the center of the protein and some distance from the vestibule. These ions are arranged along TM4's polar surface and are called polar tracks. They are 6-10 Å from aqueous solutions and have no clear aqueous pathway to external solutions.
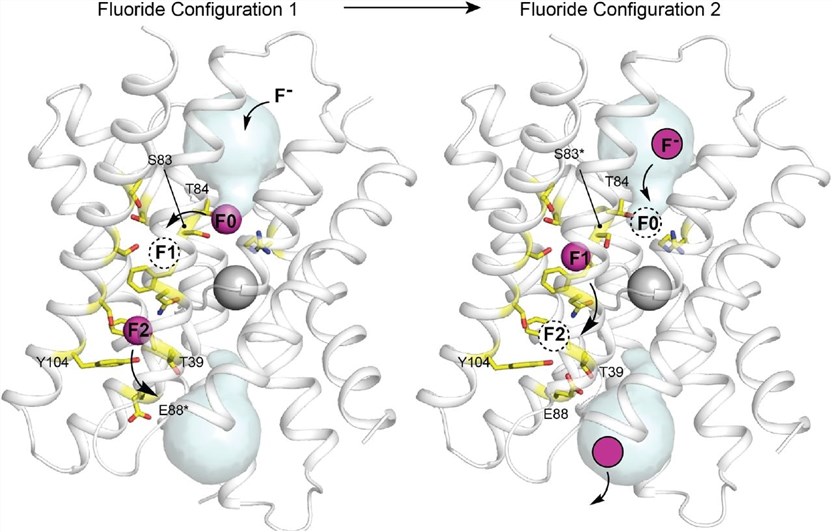 Figure 1. Multi-ion permeation mechanism for Fluc-Bpe. (Benjamin C, et al., 2021)
Figure 1. Multi-ion permeation mechanism for Fluc-Bpe. (Benjamin C, et al., 2021)
| Protein | Organism | Method | Resolution | PDB Entry ID |
|---|---|---|---|---|
| Fluoride channel Fluc-Ec2 wild-type with bromide | Escherichia coli, Homo sapiens | X-ray diffraction | 3.11 Å | 7KKR |
| Fluoride channel Fluc-Ec2 mutant S81A with bromide | Escherichia coli, Homo sapiens | X-ray diffraction | 2.50 Å | 7KKA |
| Fluoride channel Fluc-Ec2 mutant S81C with bromide | Escherichia coli, Homo sapiens | X-ray diffraction | 2.90 Å | 7KKB |
| Fluoride channel Fluc-Ec2 mutant S81T with bromide | Escherichia coli | X-ray diffraction | 2.70 Å | 7KK8 |
| Fluoride channel Fluc-Ec2 mutant S81A/T82A with bromide | Escherichia coli | X-ray diffraction | 3.10 Å | 7KK9 |
Table 1. Structural research of Fluc family fluoride channels.
Scientists have obtained high-resolution structures of these channel proteins. This was combined with X-ray crystallography, planar lipid bilayer electrophysiology, and liposome flux measurements to determine the access points to the polar orbitals. Structural studies have revealed the specific conformation and channel opening and closing mechanisms of the Fluc family channels. In addition, structural studies have revealed the subunit composition of these proteins and their key residues for binding to fluoride ions. This supports scientists to further investigate their interaction with drugs, disease-associated mutations, and novel therapeutic approaches.
Creative Biostructure is a biotechnology company focused on protein structural biology. We offer Fluc family channel structure research services. We have advanced equipment and technology to perform a wide range of structural analysis methods including X-ray crystallography, NMR, and Cryo-EM. We can help you with breakthrough protein structure research. If you are interested in our protein structure research services, please contact us for a more detailed description of our services.
Reference
- McIlwain BC, et al. The fluoride permeation pathway and anion recognition in Fluc family fluoride channels. Elife. 2021;10.