Structural Research of Heme-Handling Proteins
Heme-handling proteins play a pivotal role in various cellular processes, including respiration and photosynthesis, by facilitating electron transfer through the utilization of heme as a cofactor. Understanding the structural basis and mechanisms underlying heme binding and transport is crucial for unraveling the intricate workings of these proteins. Over the past few decades, significant progress has been made in the structural research of heme-handling proteins, shedding light on their complex functions and interactions.
Cryo-EM has revolutionized the field of structural biology, enabling researchers to visualize large macromolecular complexes in near-atomic detail without the need for crystallization. This technique has been instrumental in unraveling the intricate interactions between heme and the proteins responsible for its handling.
In a groundbreaking study, researchers successfully obtained high-resolution cryo-EM structures of the CcmABCD complex, a critical player in the transport and linkage of heme to cytochrome c proteins. The unbound form of CcmABCD, in complex with an inhibitor (AMP-PNP), and in complex with ATP and heme were all analyzed, providing valuable insights into the structural changes during heme transfer. Through their meticulous research, crucial binding sites within the CcmABCD complex are identified. The heme-binding site was localized to the CcmC subunit, which plays a central role in coordinating heme transfer. On the other hand, the ATP-binding site was pinpointed to the CcmA subunit, highlighting the significance of ATP hydrolysis in the heme transport process.
To support their findings, the researchers employed functional studies that demonstrated the hypothetical model of heme trafficking. This model proposes the transfer of heme to the periplasmic heme chaperone, CcmE, followed by the ATP-dependent release of holoCcmE from the CcmABCD complex. This detailed mechanistic insight provides a clearer understanding of how heme-handling proteins function within the cell.
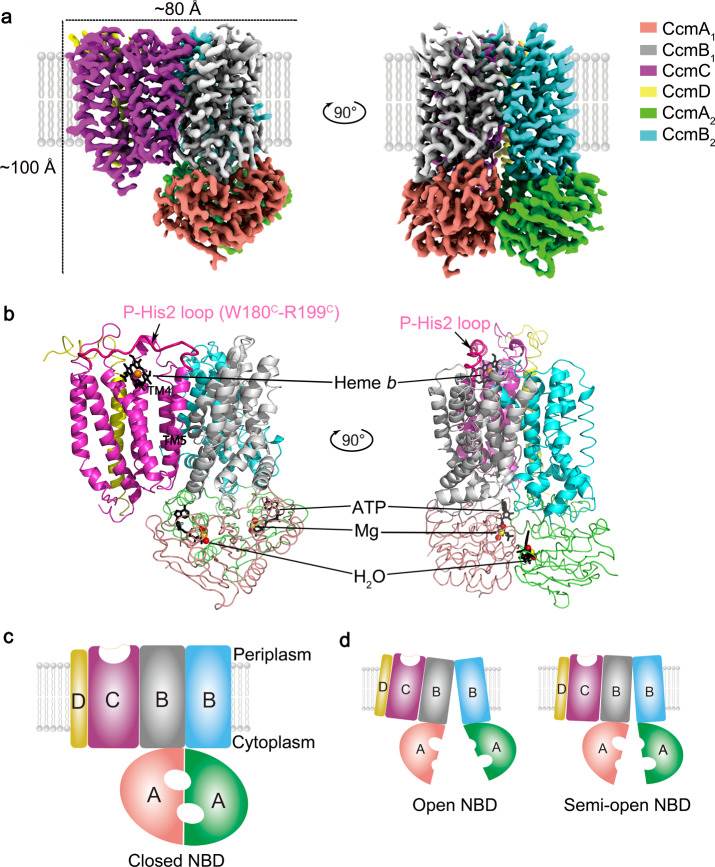 Figure 1. Overall structure of the E. coli cytochrome c maturation complex CcmABCD. (Li J, et al., 2022)
Figure 1. Overall structure of the E. coli cytochrome c maturation complex CcmABCD. (Li J, et al., 2022)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Cytochrome c Heme Lyase CcmF (expressed in Escherichia coli) | Thermus thermophilus HB27 | X-ray diffraction | 2.67 Å | 6ZMQ |
| Cytochrome c-type biogenesis protein CcmABCD, apo form, closed NBD state (expressed in Escherichia coli) | Escherichia coli | Cryo-EM single particle analysis | 3.24 Å | 7F02 |
| CcsBA bifunctional protein (heme transporter & cyt c synthase), Open Conformation (expressed in Escherichia coli) | Helicobacter hepaticus | Cryo-EM single particle analysis | 3.56 Å | 7S9Y |
Table 1. Structural research of heme-handling proteins.
As a prominent player in the structural biology arena, Creative Biostructure offers a range of services tailored to investigate heme-handling proteins and other biological molecules. Leveraging our extensive expertise and state-of-the-art technology, we provide comprehensive structural analysis services to academia and the biotechnology industry.
Our cryo-EM services have been pivotal in elucidating the structures of complex macromolecular assemblies. Our team of expert scientists utilizes advanced technologies such as cryo-EM, NMR spectroscopy and X-ray crystallography to obtain high-resolution structural data. Contact us to discover how our cutting-edge capabilities can empower your research and drive you closer to achieving your scientific goals.
References
- Brausemann A, et al. Architecture of the membrane-bound cytochrome c heme lyase CcmF. Nature Chemical Biology. 2021, 17(7): 800-805.
- Li J, et al. Structures of the CcmABCD heme release complex at multiple states. Nature Communications. 2022, 13(1): 6422.
- Mendez D L, et al. Cryo-EM of CcsBA reveals the basis for cytochrome c biogenesis and heme transport. Nature Chemical Biology. 2022, 18(1): 101-108.