Cytoskeleton Structure
The cytoskeleton is a dynamic, complex network of protein filaments found in the cytoplasm of all eukaryotic cells. It plays a critical role in maintaining cell shape, enabling cell movement, facilitating intracellular transport, and regulating cell division. Specifically, the cytoskeleton provides structural support, similar to the vertebrate skeleton, allowing cells to maintain their shape and resist mechanical stress. In addition to its structural role, the cytoskeleton is involved in cell motility through mechanisms such as cilia and flagellar movement, amoeboid movement, and muscle contraction. It plays a critical role in intracellular trafficking by providing tracks along which motor proteins such as kinesins and dyneins move organelles and vesicles within the cell. In addition, the cytoskeleton is integral to cell division, particularly during mitosis and cytokinesis, where it helps to separate chromosomes and divide the cytoplasm. The cytoskeleton is composed of three main types of protein filaments: microfilaments, microtubules, and intermediate filaments. Each type of filament has distinct structural characteristics and functions, but they work together to coordinate various cellular processes.
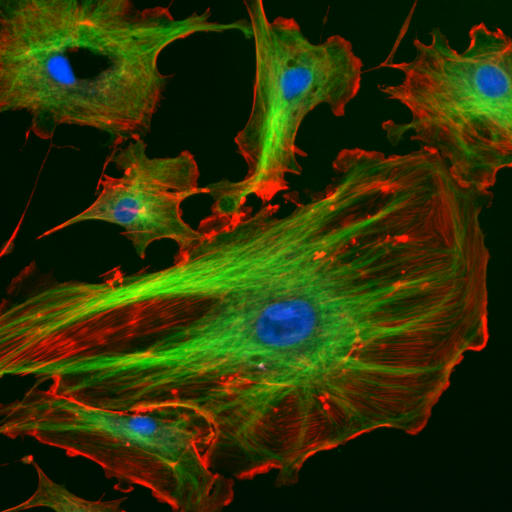 Figure 1. Fluorescence microscope image of endothelial cells – green: tubulin of microtubule, red: F-actin of filaments, blue: cell nuclei.
Figure 1. Fluorescence microscope image of endothelial cells – green: tubulin of microtubule, red: F-actin of filaments, blue: cell nuclei.
Microfilaments
Microfilaments, also known as actin filaments, are the thinnest filaments in the cytoskeleton, about 7 nanometers in diameter. They are composed primarily of actin, a globular protein that polymerizes to form long, thin, helical fibers. Actin filaments are highly dynamic, capable of rapid assembly and disassembly, allowing cells to rapidly adapt to their environment.
Microfilaments are formed by the polymerization of actin monomers (G-actin) into filamentous actin (F-actin). These filaments have an inherent polarity, with a plus (+) end where polymerization is more rapid and a minus (-) end where depolymerization is more common. This polarity is critical for the directional movement of motor proteins along the filaments. Functionally, microfilaments are involved in a variety of cellular processes, including cell shape and structure, cell movement, muscle contraction, and intracellular transport. Specifically, actin filaments form a dense network just below the plasma membrane, providing structural support and helping to maintain cell shape. Actin polymerization at the leading edge of the cell drives cell motility in processes such as amoeboid movement and the formation of lamellipodia and filopodia. In muscle cells, actin filaments interact with myosin motor proteins to generate contractile force. In addition, actin filaments serve as tracks for the transport of vesicles, organelles, and other cargo within the cell.
The dynamics of actin filaments are regulated by a variety of actin-binding proteins that control polymerization, depolymerization, and filament stability. For example, proteins such as profilin and thymosin-β4 bind to actin monomers and regulate their addition to filaments, while cofilin accelerates filament turnover by promoting depolymerization. Actin-related proteins (ARPs) and formins nucleate new filaments and promote branching, thereby increasing the complexity and functionality of the actin cytoskeleton.
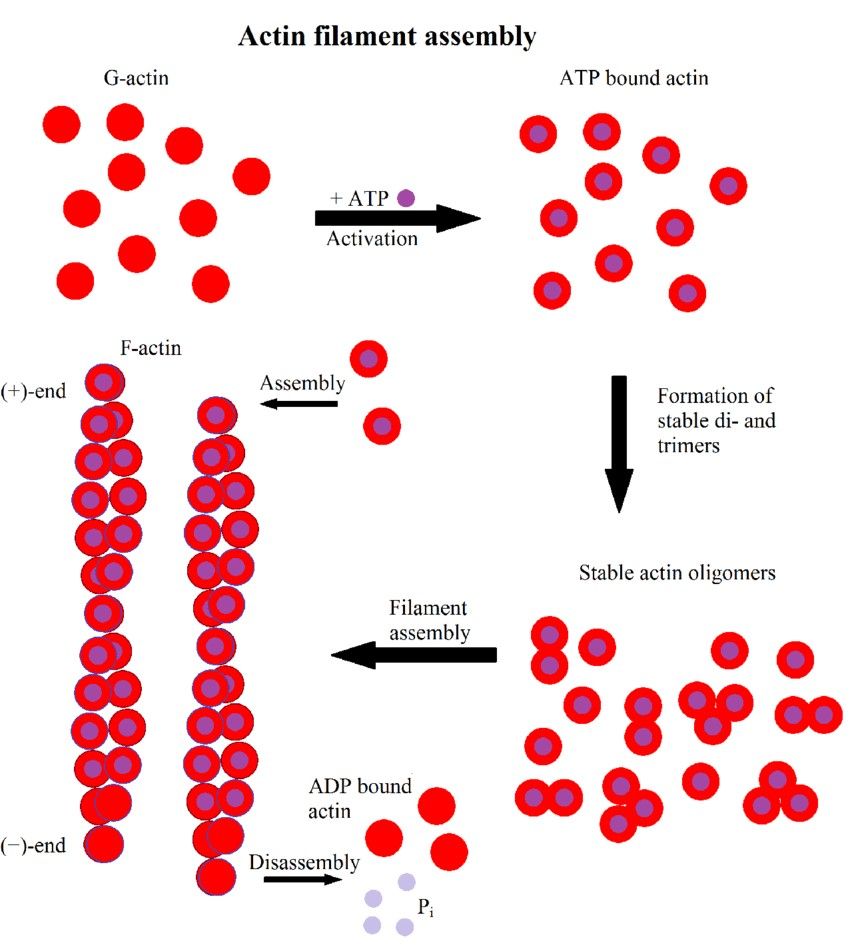 Figure 2. Scheme of actin filament formation. First, G-actin binds to ATP. It then forms stable di- or trimers and finally filaments elongate by monomer addition. Hydrolysis of ATP to ADP results in a distinction between the fast growing (+)-end and the slower growing or dissociating (-)-end (Hohmann & Dehghani,2019).
Figure 2. Scheme of actin filament formation. First, G-actin binds to ATP. It then forms stable di- or trimers and finally filaments elongate by monomer addition. Hydrolysis of ATP to ADP results in a distinction between the fast growing (+)-end and the slower growing or dissociating (-)-end (Hohmann & Dehghani,2019).
Microtubules
Microtubules (MT) are complex, dynamic, interconnected protein filaments. Together with other proteins, they perform various functions such as maintaining cell shape and polarization, controlling cell motility and intracellular transport. In addition, MTs serve as chromosome segregators during mitosis and meiosis.
MTs are polarized structures composed of α- and β-tubulin heterodimer subunits assembled into linear protofilaments. Tubulin is a small, globular protein. In cross section, MTs typically consist of a simple tube 22-25 nm in diameter, built up of 13 protofilaments around a cavity and free in the cytoplasm. In each protofilament, the αβ heterodimers are oriented so that the β-tubulin monomer faces the fast-growing end (plus-end or (+)-end) and the α-tubulin monomer is exposed at the slow-growing end (minus-end or (-)-end). MT nucleation occurs in the γ-tubulin ring complex (γ-TuRC) of the microtubule organizing center (MTOC).
The MTOC is responsible for the organization and nucleation of MT. There are different forms of MTOCs, depending on the cell type and organism. Kinetosomes and centrosomes are found in animals. In mammalian centrioles, MTs consist of a triple tube, also called microtubule triplet. The MTOC is characterized by the presence of γ-tubulin, a third tubulin isoform. γ-Tubulin and several other associated proteins together form a cone-shaped structure termed γ-TuRC. This complex serves as a template for the correct assembly of MT at the (-)-end and also as a cap of the (-)-end. This cap provides both stability and protection for the (-)-end of the MT from enzymes that could lead to its depolymerization, while inhibiting the growth of the (-)-end. MT growth continues from the MTOC towards the (+) end. The tubulin dimers, which are newly incorporated at the (+)-end by polymerization, bind GTP to the β-tubulin. The tubulin subunits hydrolyze their GTP stochastically after polymerization. This complex serves as a template for the correct assembly of the MT at the (-)-end and also as a cap of the (-)-end. This cap provides both stability and protection for the (-)-end of the MT from enzymes that could lead to its depolymerization, while inhibiting growth of the (-)-end. MT growth continues from the MTOC to the (+) end. The tubulin dimers newly incorporated by polymerization at the (+)-end bind GTP to β-tubulin. The tubulin subunits hydrolyze their GTP stochastically after polymerization.
In general, the (+)-end of the MT has a GTP tubulin cap that provides structural integrity to the MT assembly. Depolymerization of the MT occurs when the hydrolysis of GTP is faster than the binding of new GTP tubulin. This occurs when the GTP molecule in the last tubulin of the protofilament is hydrolyzed to GDP, leading to rapid depolymerization of the MT and a process known as "catastrophe". Depolymerization is interrupted only when a new GTP tubulin binds to the disintegrating filament, forming a new GTP tubulin cap. This process is called "rescue". The alternation between polymerization and depolymerization of MT is called dynamic instability.
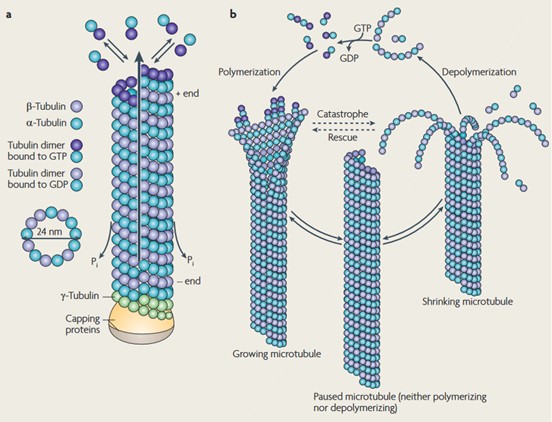 Figure 3. Structure and dynamic instability of microtubules. (a) αβ-tubulin dimers form protofilaments that normally give 13 tubular MTs. The tubulin dimers at the (+)-end, which are reconnected by polymerization, have GTP bound to the β-tubulin, which is later hydrolyzed to GDP. (b) The dynamic instability of MT. MTs undergo a continuous cycle of polymerization and depolymerization (Conde and Cáceres, 2009).
Figure 3. Structure and dynamic instability of microtubules. (a) αβ-tubulin dimers form protofilaments that normally give 13 tubular MTs. The tubulin dimers at the (+)-end, which are reconnected by polymerization, have GTP bound to the β-tubulin, which is later hydrolyzed to GDP. (b) The dynamic instability of MT. MTs undergo a continuous cycle of polymerization and depolymerization (Conde and Cáceres, 2009).
Intermediate Filaments
Intermediate filaments are so named because their diameter, about 10 nanometers, is intermediate between that of microfilaments and microtubules. Unlike the other two types of cytoskeletal filaments, intermediate filaments are not involved in cell motility, but primarily provide mechanical support to cells.
The primary functions of intermediate filaments include structural support, cell integrity, and nuclear structure. Specifically, they provide mechanical strength to cells and tissues, helping them withstand stress. Intermediate filaments also anchor organelles in place and maintain the structural integrity of the nucleus and cytoplasm. Nuclear lamins, a type of intermediate filament, form a dense network beneath the nuclear envelope, providing structural support and regulating nuclear activities.
Intermediate filaments are composed of a variety of proteins, including keratins, vimentin, desmin, and lamins, depending on the cell type. These proteins form elongated, fibrous subunits that intertwine to form strong, rope-like filaments. Intermediate filaments arise from the monomers spiraling around each other to form dimers. Two dimers aggregate to a tetramer and eight tetramers to a unit length filament. Unit filaments form the final filament via end-to-end aggregation. Notably, this process is independent of ATP or GTP. Their assembly and disassembly are regulated by phosphorylation and dephosphorylation. For example, during mitosis, phosphorylation of nuclear lamins leads to disassembly of the nuclear lamina, allowing the nuclear envelope to break down.
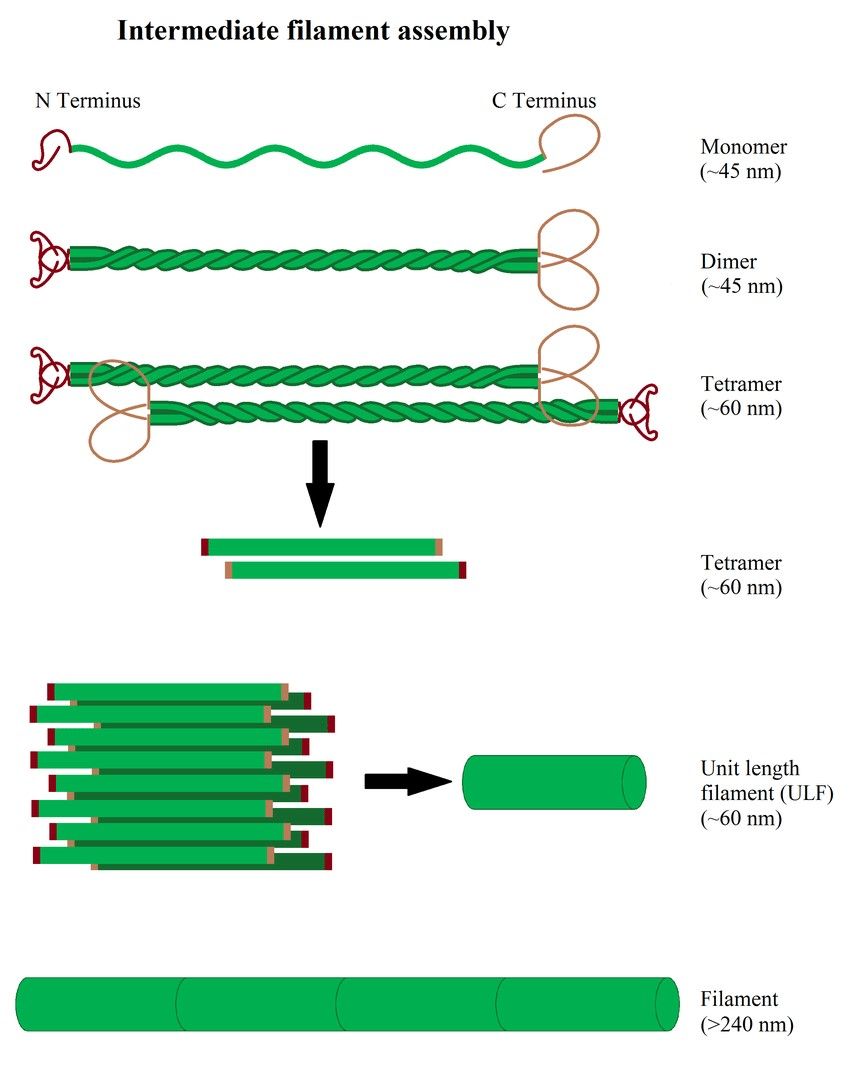 Figure 4. Illustration of intermediate filament assembly (Hohmann & Dehghani,2019).
Figure 4. Illustration of intermediate filament assembly (Hohmann & Dehghani,2019).
Interaction and Coordination among Cytoskeletal Components
The three types of cytoskeletal filaments do not function in isolation but interact and coordinate with each other to carry out complex cellular processes. These interactions are mediated by various cross-linking proteins and signaling pathways.
Cell Shape and Polarity: Actin filaments and microtubules work together to establish and maintain cell polarity, which is essential for directional cell migration and the spatial organization of cellular components.
Intracellular Transport: Microtubules and actin filaments provide complementary pathways for the transport of vesicles and organelles. For example, vesicles can move along microtubules for long-distance transport and then switch to actin filaments for short-distance movement near the cell cortex.
Mechanical Stability: Intermediate filaments provide mechanical support to the cell and help anchor microtubules and actin filaments, contributing to overall cellular integrity and resistance to mechanical stress.
Cell Division: During mitosis, microtubules form the mitotic spindle, while actin and intermediate filaments reorganize to facilitate chromosome segregation and cytokinesis.
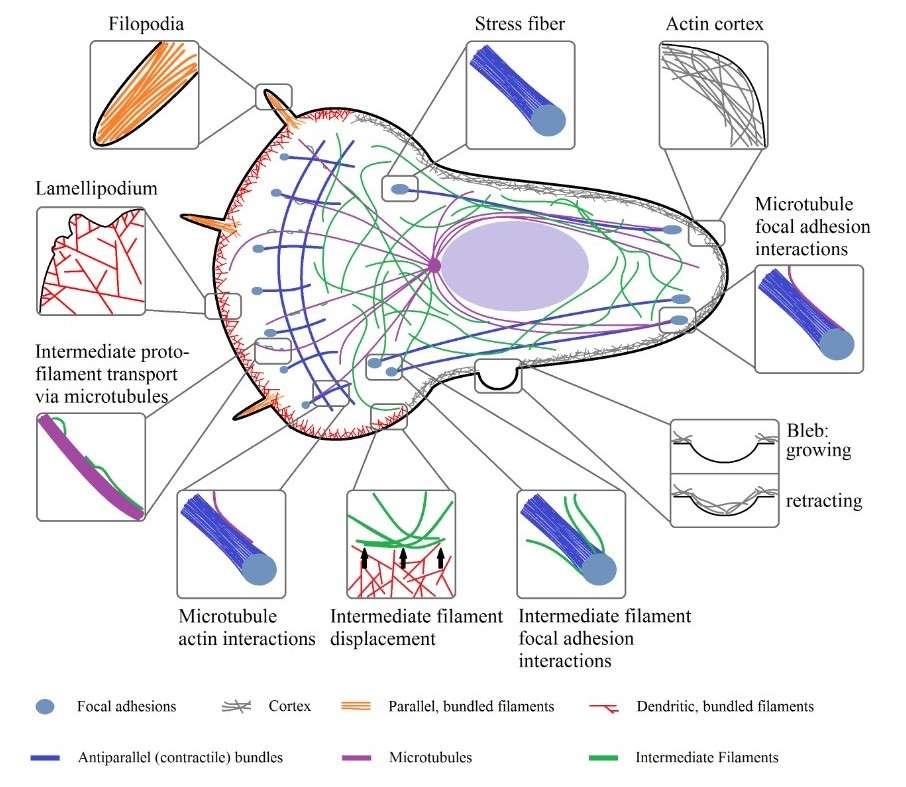 Figure 5. Organizational structures of actin, microtubules, and intermediate filaments inside of a cell and their physical interactions (Hohmann & Dehghani,2019).
Figure 5. Organizational structures of actin, microtubules, and intermediate filaments inside of a cell and their physical interactions (Hohmann & Dehghani,2019).
Methods for Cytoskeleton Analysis
Studying cytoskeletal structure involves a variety of techniques that allow researchers to visualize, quantify, and analyze the components and dynamics of the cytoskeleton. Here are some common methods used to study cytoskeletal structure:
| Techniques | Description | |
| Microscopy Techniques | Immunofluorescence | Uses antibodies specific for cytoskeletal proteins (e.g., actin, tubulin, vimentin) conjugated to fluorescent dyes to visualize cytoskeletal filaments in fixed cells. |
| Live-cell Imaging | Involves the use of fluorescently labeled cytoskeletal proteins or fluorescent tags (e.g., GFP-tubulin, LifeAct-GFP for actin) to study cytoskeleton dynamics in live cells. | |
| Super-resolution Microscopy | Techniques such as Stimulated Emission Depletion (STED), Photoactivated Localization Microscopy (PALM), and Stochastic Optical Reconstruction Microscopy (STORM) provide higher resolution images of cytoskeletal structures beyond the diffraction limit of light. | |
| Transmission Electron Microscopy (TEM) | Provides high-resolution images of cytoskeletal structures in thin cell sections, allowing detailed visualization of filament organization. | |
| Scanning Electron Microscopy (SEM) | Provides detailed surface views of cytoskeletal structures, particularly useful for studying cell morphology and filament arrangement at the cell surface. | |
| Atomic Force Microscopy (AFM) | AFM can be used to study the surface topology and mechanical properties of cytoskeletal filaments at nanometer resolution, providing insights into filament organization and interactions. | |
| Biochemical Techniques | Western Blotting | Used to detect and quantify specific cytoskeletal proteins. It involves the separation of proteins by gel electrophoresis, transfer to a membrane, and detection using specific antibodies. |
| Immunoprecipitation | Allows the isolation and analysis of cytoskeletal protein complexes by using specific antibodies to precipitate target proteins from cell lysates, followed by identification and analysis by Western blotting or mass spectrometry. | |
| Protein Purification and Reconstitution | Cytoskeletal proteins can be purified from cells or tissues and reconstituted in vitro to study their polymerization, dynamics, and interactions. This approach helps to understand the biochemical properties and assembly mechanisms of cytoskeletal filaments. | |
| Molecular and Genetic Techniques | Gene Knockdown/Knockout | RNA interference (RNAi) or CRISPR-Cas9 gene editing can be used to knock down or knock out specific cytoskeletal proteins, allowing their role in cell structure and function to be studied. |
| Overexpression and Mutagenesis | Overexpression of wild-type or mutant forms of cytoskeletal proteins can help to understand their functions and interactions. Site-directed mutagenesis can be used to identify critical regions or residues involved in filament assembly or regulation. | |
| Functional Assays | Cell Motility Assays | Techniques such as wound healing assays, transwell migration assays, and time-lapse microscopy can be used to study the role of the cytoskeleton in cell movement and migration. |
| Traction Force Microscopy | This method measures the forces exerted by cells on their substrate, providing insights into the mechanical properties of the cytoskeleton and its role in cell adhesion and motility. | |
| Microrheology | Uses small probes (e.g., beads) introduced into the cytoplasm to measure the viscoelastic properties of the cytoskeleton. This technique helps to understand the mechanical behavior of cytoskeletal networks. | |
| Advanced Techniques | Fluorescence Recovery After Photobleaching (FRAP) | FRAP involves bleaching a fluorescently labeled region of the cytoskeleton and monitoring the recovery of fluorescence over time, providing information on the dynamics and turnover of cytoskeletal proteins. |
| Förster Resonance Energy Transfer (FRET) | FRET can be used to study protein-protein interactions within the cytoskeleton by detecting energy transfer between two fluorophores in close proximity. | |
| Photoactivation and Photoconversion | These techniques involve the use of photoactivatable or photoconvertible fluorescent proteins to track the movement and dynamics of cytoskeletal components in living cells. | |
| Single-Molecule Techniques | Single molecule tracking and force spectroscopy allow the study of individual cytoskeletal filaments and their interactions with motor proteins or other binding partners at high-resolution. |
The cytoskeleton is a complex and dynamic network that is essential for multiple cellular functions. Microfilaments, intermediate filaments, and microtubules each contribute unique structural and functional properties, yet they work together to maintain cell shape, enable movement, support intracellular transport, and facilitate cell division. Understanding the intricate interactions and regulatory mechanisms of the cytoskeleton is critical to understanding cellular behavior and addressing various diseases associated with cytoskeletal dysfunction.
Creative Biostructure is dedicated to the detailed analysis of subcellular structures. We offer specialized cytoskeletal structure assay services crucial for science, medicine, and industry. Contact us for a personalized quote and see how our services can enhance your cytoskeleton research.
References
- Molecular Biology of the Cell, 4th edition.
- Conde, C., & Cáceres, A. (2009). Microtubule assembly, organization and dynamics in axons and dendrites. Nature Reviews. Neuroscience, 10(5), 319–332.
- Hohmann, T., & Dehghani, F. (2019). The cytoskeleton—A complex interacting meshwork. Cells, 8(4), 362.
- Vale, R. D. (2003). The molecular motor toolbox for intracellular transport. Cell, 112(4), 467–480.