MemPro™ HER2/ErbB2
Creative Biostructure provides custom MemPro™ gene-to-structure services for HER2/ErbB2.
Human epidermal growth factor receptor 2 (HER2/ErbB2) is a 185-kDa transmembrane glycoprotein receptor tyrosine kinase involved in the signal transduction pathways leading to cell growth and differentiation. Compared with the other three members of the epidermal growth factor receptor family (EGFR, HER3 and HER4), HER2 has a unique extracellular domain which no natural ligand was found. However, Her2 could bind with any other family members to form a heterodimer to result in receptor activation.
Amplification or over-expression of HER2 play essential role in the development of breast cancer and other epithelial malignancies. 20%-30% overexpression of HER2 is observed in breast cancers as well as ovarian, stomach, bladder, salivary and lung carcinomas. Amplified HER2/ErbB2 significantly disrupt normal cell-control mechanism, gives rise of aggressive tumors and lower the overall survival rate of patients. The heterodimerization of HER2 and HER3 could continuously activate downstream signaling pathways such as MAPK, PI3K and PKB through phosphorylation of the tyrosine kinases. It accelerate the cell proliferation process and help bypass the check points and results in tumor formation. The important roles of ErbB2 in cancer progression render it a highly attractive target for therapeutic interventions of breast cancer.
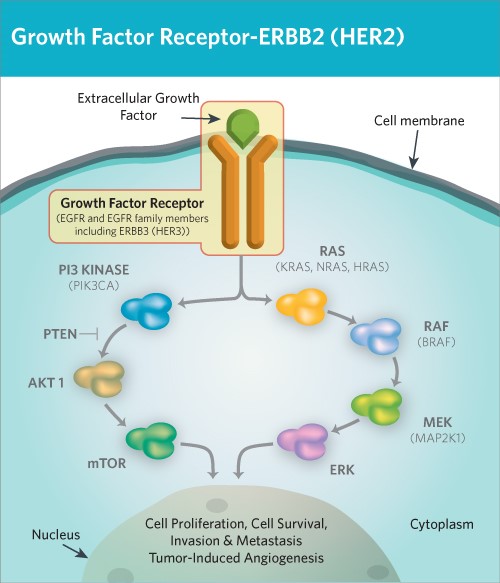 Figure 1. HER2/ErbB2 induce cell proliferation
Figure 1. HER2/ErbB2 induce cell proliferation
Trastuzumab (Herceptin), a monoclonal antibody that interferes with the HER2/neu receptor, was approved by FDA in 1998. It not only binds to the extracellular domain of ErbB2, but also shows additional mechanisms such as activation of the PTEN tumor suppressor gene, induction of p27 and induction of G1 cell cycle arrest. Treatment of ErbB2-overexpressing cells with trastuzumab can inhibit ErbB2-mediated Cdc2-Tyr15 phosphorylation and p21 upregulation, which allows effective p34 activation and induction of apoptosis upon paclitaxel treatment. The combination of trastuzumab and chemotherapy produced significantly greater clinical benefit to overcome chemoresistance. ErbB2 over-expression breast cancer cells are intrinsically resistant to DNA-damaging agents and drug-induced apoptosis is more likely to fail.
Pertuzumab (2C4), approved in 2013, is also a monoclonal antibody that associated with breast cancer treatment. Different with trastuzumab which could only bind to homodimerization of HER2, pertuzumab specifically blocks dimerization domain of HER2 to inhibit HER2 to heterodimerize with other EGFR family members. In patients with HER2-positive metastatic breast cancer, the addition of pertuzumab to trastuzumab and docetaxel, as compared with the addition of placebo, significantly improved the median overall survival to 56.5 months and extended the results of previous analyses showing the efficacy of this drug combination.
Affibody molecules were designed as an alternative to immunoglobulin-based binding proteins. The parental “Z domain” is a small protein of 58 amino acids organized into a three-helix bundle. It was developed as a stabilized variant of the “B domain” of the IgG-binding staphylococcal protein A. The high affinity of ZHER2 for its HER2 target is a result of so-called affinity maturation, which is a second round of combinatorial protein engineering and phage display selection. ZHER2 competes with neither trastuzumab nor pertuzumab for HER2 binding. These properties make it suitable as a tracer when the aim is to study HER2 without affecting its function, even in the presence of therapeutic monoclonal antibodies.
References:
Eigenbrot C, Ultsch M, Dubnovitsky A, et al. Structural basis for high-affinity HER2 receptor binding by an engineered protein[J]. Proceedings of the National Academy of Sciences, 2010, 107(34): 15039-15044.
Tan M, Yu D. Molecular mechanisms of erbB2-mediated breast cancer chemoresistance[M]//Breast Cancer Chemosensitivity. Springer New York, 2007: 119-129.