Structural Research of Mechanosensitive Channels
Mechanosensitive channels (MSC) are membrane proteins capable of responding to mechanical stress in response to external mechanical stimuli. In living organisms, MSC is used to sense mechanical stimuli and convert physical stresses on cell membranes into electrochemical reactions that enable them to respond accordingly.
Research Progress on the Structure of MSC in Prokaryotes
In prokaryotes, the small conductance mechanosensitive channels MScS are homoheptamers, each subunit containing three transmembrane domains, with the n end facing the membrane and the c end embedded in the cytoplasm. Large conductivity force-sensitive channel (MScL) is a homopentameter. It is mainly composed of helical regions opposite the bilayer. Contains two domains. In the MScL structure, the helix passes through the membrane twice at the C and N ends.
Research Progress on the Structure of MSC in Eukaryotes
Among eukaryotes, the TRP protein is the best-known MSC. The TRP protein has a pore between the transmembrane domains S5 and S6. They contain intracellular N and C ends, forming a tetramer. TRP proteins mediate protein interactions and help stretch activation channels open.
X-ray crystallography and low-temperature electron microscopy have been used to study the structure of MSC Proteins. The structural information obtained from experiments can help scientists gain insight into the response to mechanical stress mechanisms in living organisms.
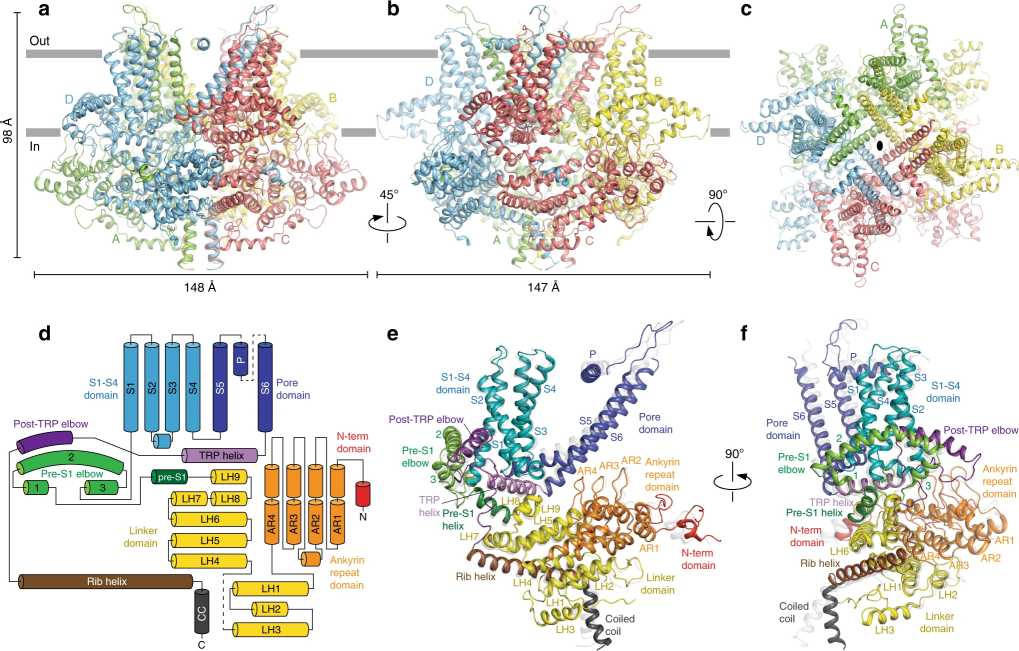 Figure 1. Architecture and domain organization of crTRP1. (McGoldrick L L, et al., 2019)
Figure 1. Architecture and domain organization of crTRP1. (McGoldrick L L, et al., 2019)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Thermo-Sensitive TRP Channel TRP1 from the Alga Chlamydomonas reinhardtii | Chlamydomonas reinhardtii | Cryo-EM single particle analysis | 3.45 Å | 6PW5 |
| TRPC3 in a lipid-occupied, closed state | Homo sapiens | Cryo-EM single particle analysis | 3.30 Å | 6CUD |
| Canonical TRPC4 ion channel | Danio rerio | Cryo-EM single particle analysis | 3.60 Å | 6G1K |
| Human TRPC6 | Homo sapiens | Cryo-EM single particle analysis | 3.80 Å | 5YX9 |
| Coiled-coil domain of the transient receptor potential channel from Gibberella zeae (TRPGz) | Fusarium graminearum PH-1 | X-ray diffraction | 1.25 Å | 3VVI |
| TRPV6 ankyrin repeat domain | Mus musculus | X-ray diffraction | 1.70 Å | 2RFA |
| Ankyrin repeat domain of human TRPV2 | Homo sapiens | X-ray diffraction | 1.70 Å | 2F37 |
| Cold- and menthol-sensing ion channel TRPM8 | Ficedula albicollis | Cryo-EM single particle analysis | 4.10 Å | 6BPQ |
| TRPML3 ion channel | Callithrix jacchus | Cryo-EM single particle analysis | 2.94 Å | 5W3S |
| Small-molecule modulation of acid-sensing ion channel 1 (ASIC1) | Gallus gallus | X-ray diffraction | 3.01 Å | 6X9H |
| Zebrafish two pore domain K+ channel TREK1 (K2P2.1) in DDM/POPA mixed micelles | Danio rerio | Cryo-EM single particle analysis | 2.82 Å | 8DE8 |
| Human two pore domain potassium ion channel TREK2 (K2P10.1) | Homo sapiens | X-ray diffraction | 3.20 Å | 4BW5 |
| Human ASIC1a-Nb.C1 complex | Homo sapiens | Cryo-EM single particle analysis | 2.86 Å | 7RNN |
Table 1. Structural research of mechanosensitive channels.
Structural analysis of mechanosensitive channels using cryo-electron microscopy (cryo-EM) and X-ray crystallography are beneficial for further research of their functions. Creative Biostructure has expertise and experience in membrane protein research. We offer cryo-EM services to determine the structure of membrane proteins using single particle analysis (SPA).
In addition to the structural determination of membrane proteins, we can also accurately analyze nucleic acids, small proteins, ribosomes, protein complexes, protein-ligand complexes, and viruses. If you are interested in our services, please contact us.
References
- Geoffrey Chang, et al. Structure of the MscL Homolog from Mycobacterium tuberculosis: A Gated Mechanosensitive Ion Channel. Science, 1998, 282:2220-2226.
- Del Valle ME, et al. Mechanosensory neurons, cutaneous mechanoreceptors, and putative mechanoproteins. Microsc Res Tech. 2012 ,75(8):1033-1034.
- McGoldrick L L, et al. Structure of the thermo-sensitive TRP channel TRP1 from the alga Chlamydomonas reinhardtii. Nature Communications, 2019, 10(1): 4180.