Structural Research of Alternanthera Mosaic Virus
Alternanthera mosaic virus (AltMV) is a filamentous plant virus belonging to the genus Potexvirus. The virus contains single-stranded RNA and capsid protein (CP), which can self-assemble into flexible spiral sheaths around the RNA. Since plant viruses and virus-like particles (VLPs) are generally safe for humans, they have the potential to achieve technological advancements in a wide range of fields, from microelectronics to the development of candidate vaccines and adjuvants. The successful application of AltMV virus particles and VLP in biotechnology has significant advantages.
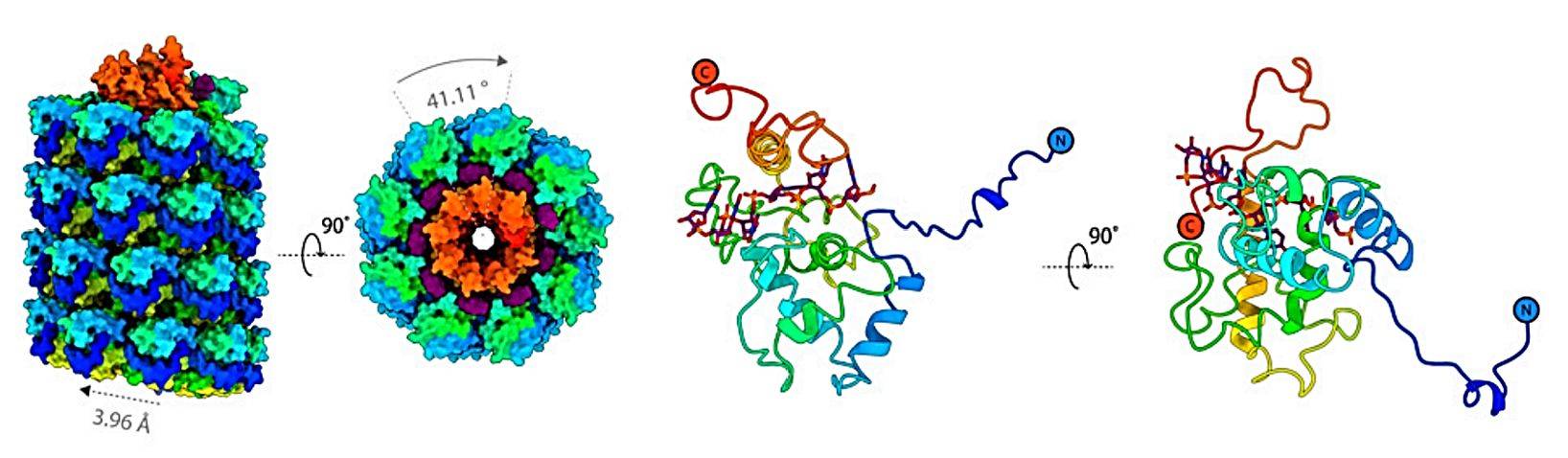 Figure 1. The cryo-EM structure of AltMV VLP. (Thuenemann EC, et al., 2021)
Figure 1. The cryo-EM structure of AltMV VLP. (Thuenemann EC, et al., 2021)
Structure of AltMV Genome
The genome of AltMV is composed of a single positive-sense, single-stranded RNA molecule that possesses a cap structure at the 5' terminus and a polyA sequence at the 3' terminus. Through nucleotide sequence analysis, the AltMV-PA genome has been found to contain two untranslated regions (UTRs) located at the 5' (1-94nt) and 3' termini (6481-6607nt) respectively. Additionally, the genome harbors five open reading frames (ORFs). The first ORF, positioned at the 5' end of the genome, is the longest and can encode a 1540aa protein known as the viral replicase (RdRp). Subsequently, the following three ORFs are presumed to encode the Triple Gene Block Proteins, which are movement proteins: ORF2 encodes 26kDa, 232aa proteins; ORF3 encodes 12kDa, 110aa protein; and ORF4 encodes 7kDa, 63aa protein. Finally, at the extreme 3' end of the genome lies ORF5. Translation of ORF5 results in the production of 22-23kDa, 207aa polypeptides, which represents the CP.
Research Progress on AltMV Virus Structure
The study of the structure of plant viruses is of great significance in reducing the damage caused by these agricultural pathogens and supporting their biotechnology applications. Nowadays, X-ray crystallography, nuclear magnetic resonance spectroscopy, and cryo-electron microscopy (cryo-EM) are recognized methods for obtaining the best resolution of 3D protein structures. Small-angle X-ray scattering (SAXS) and atomic force microscopy (AFM) have been widely used for large and complex supramolecular structures such as plant viruses, especially flexible filamentous supramolecular structures like AltMV. The identified strain AltMV-MU virus particles are spiral filamentous particles with a length of 570nm and a diameter of 13nm. AltMV CP self-assembles into VLP in vitro without viral RNA. Through cryoelectron microscopy, it was confirmed that the diameter of AltMV VLP is larger than that of the AltMV viral body, and there are more CP subunits in VLP.
The Application Prospects of AltMV
Plant viruses and VLP have been widely used in biotechnology, including the development of medical products (vaccines). The use of plant viruses as the foundation of medical nanotechnology can reduce many risks associated with other biomaterials used in vaccines, and the use of noninfectious VLPs can reduce risks to the environment and humans. These lower risks make virus nanoparticles easier to handle, transport, and process, making plant virus-based particles a particularly attractive platform for a range of nanotechnology applications. The study of the structure and stability of AltMV virus particles and VLP is a promising and very important research direction.
Creative Biostructure is a leading supplier of virus-like particles (VLPs), offering a range of high-purity products to help clients explore the potential of plant viruses in drug delivery and vaccine development.
| Cat No. | Product Name | Virus Family | Source | Composition |
| CBS-V101 | AltMV VLP | Alphaflexiviridae | Mammalian cell recombinant | AltMV coat protein |
| Explore All Alternanthera Mosaic Virus VLP Products | ||||
In addition, based on our advanced electron microscopy (EM) platform, Creative Biostructure can support researchers, pharmaceutical companies, and diagnostic professionals to deepen the understanding of viral structure and action mechanisms and contribute to developing effective vaccine interventions. Please feel free to contact us for a detailed quote.
References
- Thuenemann EC, et al. A Replicating Viral Vector Greatly Enhances Accumulation of Helical Virus-Like Particles in Plants. Viruses. 2021. 13(5): 885.
- Shtykova EV, et al. Structural Insights into Plant Viruses Revealed by Small-Angle X-ray Scattering and Atomic Force Microscopy. Viruses. 2024. 16(3): 427.
- Manukhova TI, et al. Thermal remodeling of Alternanthera mosaic virus virions and virus-like particles into protein spherical particles. PLoS One. 2021. 16(7): e0255378.
- Donchenko E, et al. Alternanthera mosaic potexvirus: Several Features, Properties, and Application. Adv Virol. 2018. 2018: 1973705.
- Donchenko EK, et al. Structure and properties of virions and virus-like particles derived from the coat protein of Alternanthera mosaic virus. PLoS One. 2017. 12(8): e0183824.