Structural Research of G Protein-coupled Receptors (GPCRs) Class F
G protein-coupled receptors (GPCRs) are a large family of membrane proteins that play crucial roles in cell signaling. Among the GPCRs, the Class F GPCRs, also known as Frizzled-Class receptors, have garnered significant attention due to their involvement in various biological processes, including development, cell proliferation, and cancer. Understanding the structural details of Class F GPCRs, particularly their activation mechanisms and interaction with signaling partners, is of utmost importance for advancing our knowledge of these receptors and developing potential therapeutic strategies.
One notable advancement in the field of Class F GPCR research has been the elucidation of the Hedgehog (HH) signaling pathway, where the oncoprotein Smoothened (SMO) acts as a Class-F GPCR. In a groundbreaking study, researchers aimed to unravel the mechanisms underlying SMO modulation by the tumor suppressor Patched-1 (PTCH1) and the involvement of G protein coupling in GLI activation. The study revealed the identification of 24,25-epoxycholesterol (24,25-EC) as an endogenous ligand of PTCH1. The researchers demonstrated that 24,25-EC stimulates HH signaling and triggers G protein signaling via human SMO (hSMO). To further understand the structural basis of this interaction, a cryo-EM structure of 24(S),25-EC-bound hSMO in complex with a heterotrimeric Gi protein was obtained. This structure provided crucial insights into the ligand binding site of 24(S),25-EC within the 7-transmembrane region (7-TMs) of hSMO and revealed a unique arrangement of the Gi protein when compared to Class-A GPCR-Gi complexes.
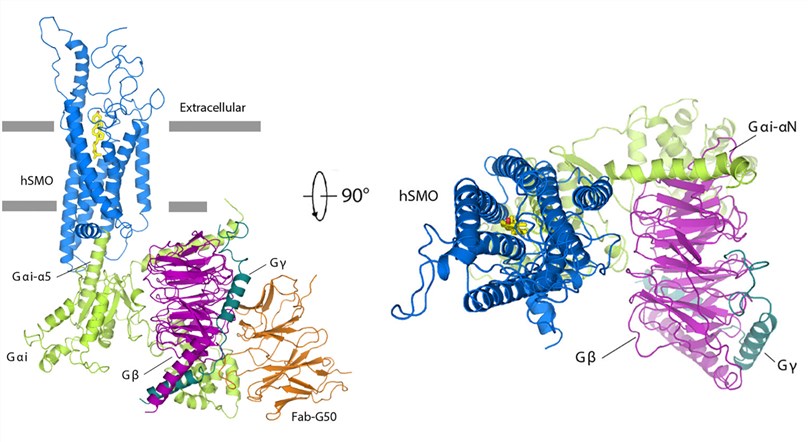 Figure 1. Structure of hSMO-Gi-Fab complex. (Qi X, et al., 2019)
Figure 1. Structure of hSMO-Gi-Fab complex. (Qi X, et al., 2019)
| Protein | Organism | Method | Resolution | PDB Entry ID |
|---|---|---|---|---|
| Smoothened 7TM receptor in complex with an antitumor agent (expressed in Spodoptera frugiperda) | Escherichia coli, Homo sapiens | X-ray diffraction | 2.45 Å | 4JKV |
| Smoothened receptor in complex with SANT-1 (expressed in Spodoptera frugiperda) | Shigella flexneri 5 str. 8401, Homo sapiens | X-ray diffraction | 2.80 Å | 4N4W |
| Smoothened (SMO) receptor in complex with cyclopamine (expressed in Spodoptera frugiperda) | Homo sapiens, Escherichia coli | X-ray diffraction | 3.20 Å | 4O9R |
| Smoothened (SMO) receptor in complex with cholesterol (apo-SMOΔC) (expressed in HEK-293S-GnTI-) | Homo sapiens, Escherichia coli | X-ray diffraction | 3.20 Å | 5L7D |
| Smoothened (SMO) receptor (multi-domain) in complex with TC114 (expressed in sf9 cells) | Homo sapiens, Desulfovibrio vulgaris str. Hildenborough | X-ray diffraction | 3.00 Å | 5V57 |
| Smoothened (SMO) receptor with bound oxysterol coupled to a heterotrimeric Gi (expressed in HEK293s cells) | Homo sapiens, Mus musculus | Cryo-EM single particle analysis | 3.84 Å | 6OT0 |
| Smoothened (SMO) receptor+Gi in complex with SAG (expressed in HEK293 cells) | Homo sapiens, Mus musculus | Cryo-EM single particle analysis | 3.96 Å | 6XBL |
| Smoothened (SMO) receptor bound to SAG21k, cholesterol, and NbSmo8 (expressed in Homo sapiens) | Mus musculus, Lama glama | X-ray diffraction | 2.80 Å | 6O3C |
| Smoothened (SMO) receptor-PGS2 in a lipidic environment, unliganded (expressed in HEK293 cells) | Mus musculus, Pyrococcus abyssi GE5 | Cryo-EM single particle analysis | 3.70 Å | 8CXO |
| Frizzled 4 (FZD 4) receptor in the ligand-free state (expressed in Spodoptera frugiperda) | Homo sapiens, Clostridium pasteurianum | X-ray diffraction | 2.40 Å | 6BD4 |
| Frizzled 5 (Fzd5) receptor in the ligand-free state (expressed in Escherichia coli, Homo sapiens) | Homo sapiens, synthetic construct, Escherichia coli | Cryo-EM single particle analysis | 3.70 Å | 6WW2 |
| Frizzled 7 (FZD 7) receptor in complex with heterotrimeric Gs (expressed in Escherichia coli, Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 3.22 Å | 7EVW |
Table 1. Structural Research of Class-F GPCRs.
As the field of structural biology continues to advance, unraveling the complexities of Class F GPCRs holds immense promise for understanding fundamental biological processes and developing targeted therapies. Creative Biostructure invites researchers and pharmaceutical companies to collaborate and utilize our exceptional structural analysis services. With years of experience in the industry, we have pioneered innovative approaches and cutting-edge techniques for structural analysis of these receptors. Our team of expert scientists utilizes advanced technologies such as cryo-electron microscopy (cryo-EM) and X-ray crystallography to obtain high-resolution structural data, providing invaluable insights into the architecture and function of Class F GPCRs.
Whether your research focuses on unraveling the mysteries of Class-F GPCR structure or developing innovative therapies for cancers, we are your premier partner. Contact us to discover how our cutting-edge capabilities can empower your research and drive you closer to achieving your scientific goals. Let us be your catalyst for progress.
References
- Qi X, et al. Cryo-EM structure of oxysterol-bound human Smoothened coupled to a heterotrimeric Gi. Nature. 2019, 571(7764): 279-283.
- Xu L, et al. Cryo-EM structure of constitutively active human Frizzled 7 in complex with heterotrimeric Gs. Cell Research. 2021, 31(12): 1311-1314.
- Zhang K, et al. Fusion protein strategies for cryo-EM study of G protein-coupled receptors. Nature Communications. 2022, 13(1): 4366.