Structural Research of Mitochondrial Outer Membrane Beta Barrel Proteins
Mitochondrial outer membrane beta barrel proteins mediate the physiological pathway of protein entry into mitochondria, metabolite transport and lipid transport. It contains three protein families: Sam50, Tom40 and VDAC. Together with Mdm10, they form a group of β-barrel proteins on the outer of the mitochondrial membrane. The sorting assembly machine (SAM) is the core of β-barrel protein in the mitochondrial outer membrane. SAM consists of 16 chains Sam50, Sam35, and Sam37.
Research Progress of SAM Composite Structures
In the lipid nanodisk, the SAM complex is a monomer. Sam50 forms a 16-strand transmembrane β-barrel structure in which the POTRA domain extends into the intermembrane space. Sam35 and Sam37 are located on the cytoplasmic side of the cell membrane. Sam35 covers the lumen of Sam50. In detergent, the SAM complex forms a dimer. The first transmembrane alpha-helix of Sam37 passes through the membrane near the Sam50β barrel but does not contact the β barrel.
Functional and Structural Analysis of VDAC Proteins
The VDAC has been involved in the transport of mitochondria. There are three subtypes of mitochondria in mammals (hVDAC1, hVDAC2, and hVDAC3). VDAC1 may be involved in the binding of hexokinase subtypes I and II on the mitochondrial surface, bringing ATP produced by mitochondria into the cellular metabolic pathway. The VDAC model is expected to consist of 12 to 19 chains. The N-terminal helix is positioned in the middle of the barrel hole, and the aperture is changed by about 40% relative to the non-blocked form.
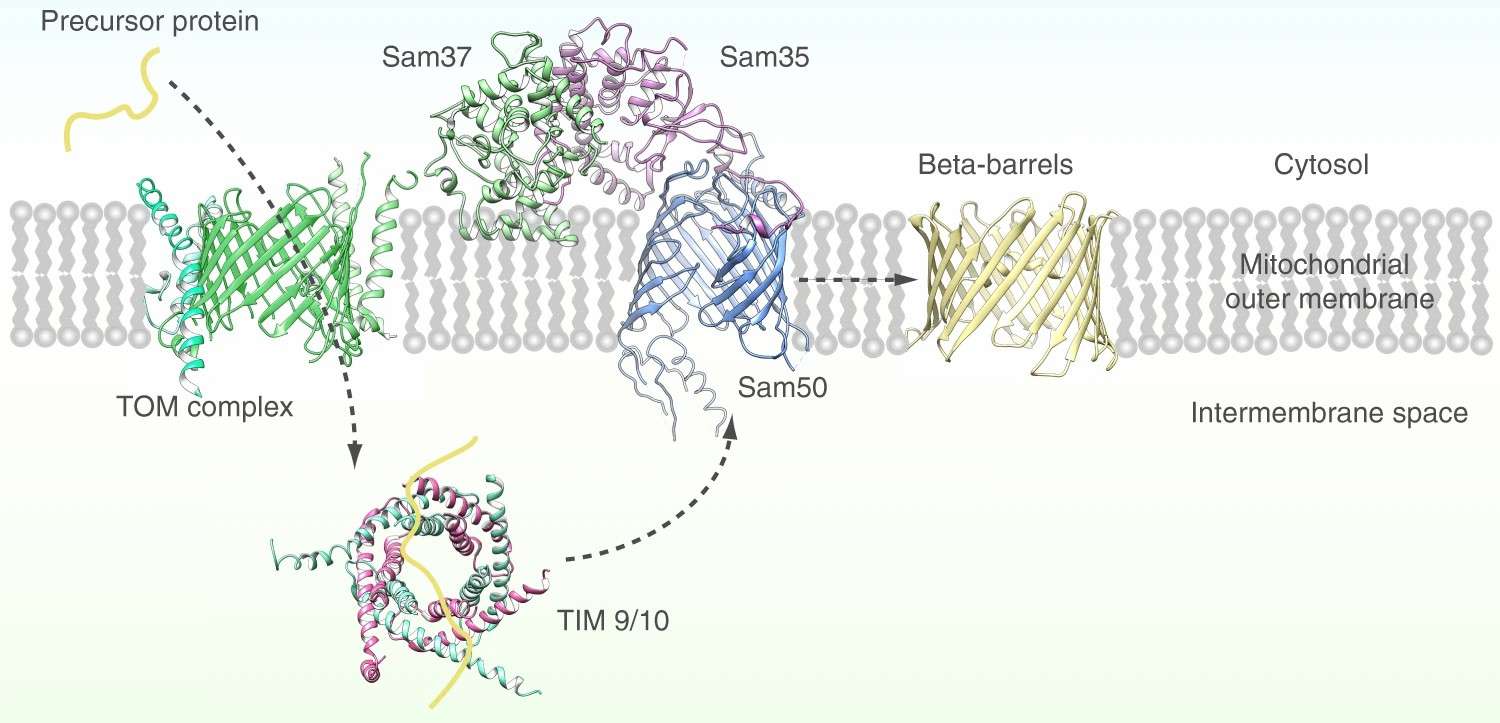 Figure 1. Mitochondrial outer membrane beta barrel proteins formation process. (Diederichs KA, et al., 2020)
Figure 1. Mitochondrial outer membrane beta barrel proteins formation process. (Diederichs KA, et al., 2020)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| The mitochondrial SAM complex | Saccharomyces cerevisiae | Cryo-EM single particle analysis | 2.90 Å | 7BTW |
| The mitochondrial SAM-Mdm10 supercomplex in GDN micelle | Saccharomyces cerevisiae | Cryo-EM single particle analysis | 2.80 Å | 7BTX |
| The mitochondrial SAM-Mdm10 supercomplex in Nanodisc | Saccharomyces cerevisiae | Cryo-EM single particle analysis | 3.20 Å | 7BTY |
| Mitochondrial SAM complex - monomer in detergent | Thermothelomyces thermophilus | Cryo-EM single particle analysis | 3.70 Å | 6WUJ |
| Mitochondrial SAM complex - dimer 3 in detergent | Thermothelomyces thermophilus | Cryo-EM single particle analysis | 3.90 Å | 6WUN |
| Mitochondrial SAM complex in lipid nanodiscs | Thermothelomyces thermophilus | Cryo-EM single particle analysis | 3.40 Å | 6WUH |
| Mitochondrial SAM complex - high-resolution monomer in detergent | Thermothelomyces thermophilus | Cryo-EM single particle analysis | 3.00 Å | 6WUT |
| The translocator of the outer mitochondrial membrane | Saccharomyces cerevisiae S288C | Cryo-EM single particle analysis | 3.81 Å | 6JNF |
| SAM-Tom40 intermediate complex | Saccharomyces cerevisiae S288C | Cryo-EM single particle analysis | 3.20 Å | 7VKU |
| Mitochondrial TOM complex | Saccharomyces cerevisiae S288C | Cryo-EM single particle analysis | 3.06 Å | 6UCU |
| Protein translocase of mitochondria | Homo sapiens | Cryo-EM single particle analysis | 3.40 Å | 7CK6 |
| TOM core complex | Neurospora crassa | Cryo-EM single particle analysis | 6.80 Å | 5O8O |
Table 1. Structural research of mitochondrial outer membrane beta barrel proteins.
To study the structure of mitochondrial outer membrane beta barrel proteins, cryo-electron microscopy (cryo-EM) is commonly used. Structural analysis of membrane proteins is beneficial to the study of biomolecular functions. It also helps in the development of new targeted drugs.
Creative Biostructure has long been committed to the study of structural biology and membrane proteins. We have extensive experience in determining membrane protein structures using single particle analysis (SPA).
In addition to the structural determination of membrane proteins, we can accurately analyze biomolecules, including but not limited to nucleic acids, ribosomes, small proteins, protein complexes, protein-ligand complexes, and viruses. If you are interested in our services, please contact us and we will provide you with a professional and comprehensive solution.
References
- Diederichs KA, et al. Structural insight into mitochondrial β-barrel outer membrane protein biogenesis. Nat Commun. 2020 ,11(1):3290.
- Zeth K. Structure and evolution of mitochondrial outer membrane proteins of beta-barrel topology. Biochim Biophys Acta. 2010, 1797(6-7):1292-1299