Structural Research of Gap Junctions and Related Channels
Gap junctions play a crucial role in facilitating intercellular communication, enabling the direct passage of signals between neighboring cells. These gap junctions are composed of connexin proteins, a family of transmembrane proteins that form intercellular channels. The connexins, with 21 isoforms identified in humans, are the key players in establishing intercellular channels for cell-to-cell communication. These channels allow the passage of electrical and small-molecule signals, including ions, second messengers, hormones, metabolites, and even therapeutic agents. Through the connexin channels, neighboring cells can exchange information, synchronize their activities, and maintain tissue homeostasis.
Significance of Lipid Environment
The lipid environment surrounding connexin channels has emerged as a critical factor influencing their structure and function. Research has shown that connexins, such as Cx46/50, have a remarkable influence on the local lipid environment. In a CryoEM study, it was revealed that Cx46/50 induces a phase separation specific to the extracellular lipid leaflet of the opposing membranes. This phase separation, shifting the lipid environment to the gel state, contributes to the stabilization and assembly of gap junctions. Molecular dynamics simulations further elucidated the heterogeneity of lipid configurations within the dynamic lattice of stabilized lipids.
Implications for Biological Processes
The structural research on connexin channels and gap junctions has broad implications for our understanding of various biological processes. The establishment of near-atomic level details for these membrane channels allows us to comprehend the molecular basis of intercellular communication and its role in cellular physiology. The water molecules resolved in the CryoEM map, localized at critical sites, suggest their involvement in the architecture and function of gap junctions. These findings highlight the complex interplay between protein-lipid interactions, water molecules, and connexin channels, shedding light on the mechanisms underlying gap junctional coupling.
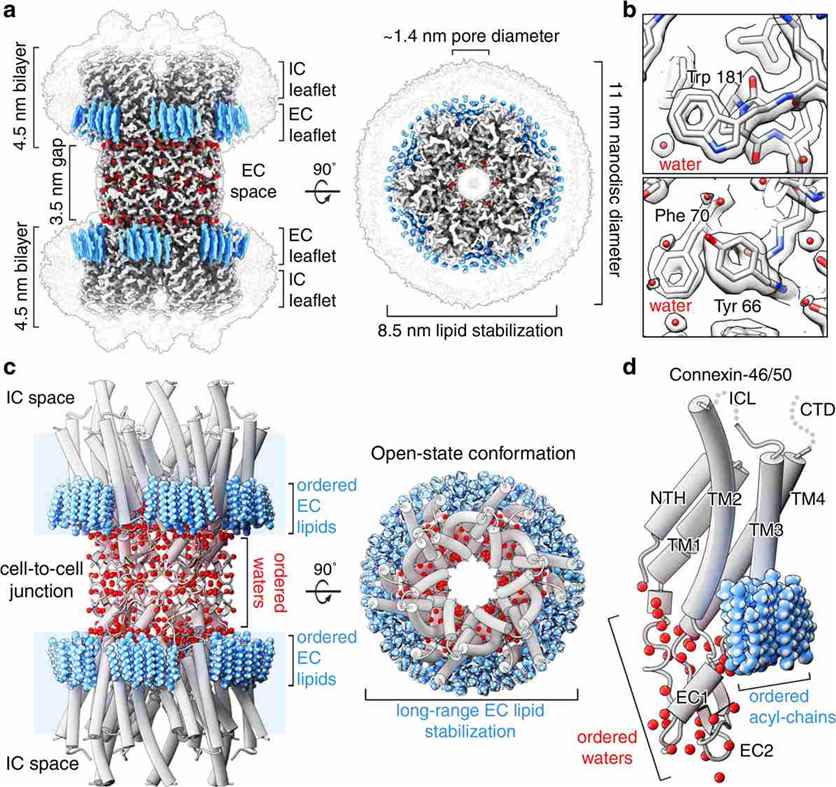 Figure 1. Structure of connexin-46/50 in lipid nanodiscs by Cryo-EM. (Flores J A, et al., 2020)
Figure 1. Structure of connexin-46/50 in lipid nanodiscs by Cryo-EM. (Flores J A, et al., 2020)
| Protein | Organism | Method | Resolution | PDB Entry ID |
|---|---|---|---|---|
| Connexin-26 gap junction channel (expressed in Sf9 cells) | Homo sapiens | X-ray diffraction | 3.50 Å | 2ZW3 |
| Connexin 26 dodecamer at 20mmHg PCO2, pH7.4 (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 2.10 Å | 7QET |
| Cx31.3/GJC3 connexin hemichannel in the absence of calcium (expressed in HEK293 cells) | Homo sapiens | Cryo-EM single particle analysis | 2.34 Å | 6L3T |
| INX-6 gap junction channel (expressed in Spodoptera frugiperda) | Caenorhabditis elegans | Cryo-EM single particle analysis | 3.60 Å | 5H1R |
| Undocked INX-6 hemichannel in a nanodisc (expressed in Spodoptera frugiperda) | Caenorhabditis elegans | Cryo-EM single particle analysis | 3.80 Å | 6KFF |
| Connexin (Cx)-46/50 intercellular gap junction channels. Cx46 channel | Ovis aries | Cryo-EM single particle analysis | 3.40 Å | 6MHQ |
| Connexin (Cx)-46/50 intercellular gap junction channels in nanodiscs. Cx-50 | Ovis aries | Cryo-EM single particle analysis | 1.94 Å | 7JJP |
| Calcium homeostasis modulator (CALHM1) voltage-gated ATP release channel (expressed in Spodoptera frugiperda) | Aequorea victoria, Gallus gallus | Cryo-EM single particle analysis | 3.63 Å | 6VAM |
| Calcium homeostasis modulator (CLHM-1) voltage-gated ATP release channel (expressed in HEK293 cells) | Caenorhabditis elegans | Cryo-EM single particle analysis | 3.60 Å | 6LMV |
| Calcium homeostasis modulator (CLHM-1) voltage-gated ATP release channel (expressed in HEK293 cells) | Caenorhabditis elegans | Cryo-EM single particle analysis | 3.73 Å | 6LOM |
| Calcium homeostasis modulator (CALHM1) voltage-gated ATP release channel (expressed in HEK293 cells) | Oryzias latipes | Cryo-EM single particle analysis | 2.66 Å | 6LMT |
| Calcium homeostasis modulator (CALHM1) voltage-gated ATP release channel (expressed in HEK293 cells) | Danio rerio | Cryo-EM single particle analysis | 3.10 Å | 6LYG |
| Calcium homeostasis modulator (CALHM2) voltage-gated ATP release channel, open state (expressed in HEK293 cells) | Homo sapiens | Cryo-EM single particle analysis | 3.30 Å | 6UIV |
| Calcium homeostasis modulator (CALHM2) voltage-gated ATP release channel (expressed in Spodoptera frugiperda) | Homo sapiens | Cryo-EM single particle analysis | 3.48 Å | 6VAK |
| Calcium homeostasis modulator (CALHM4), decameric in absence of Ca2+ (expressed in HEK293 cells) | Homo sapiens | Cryo-EM single particle analysis | 4.07 Å | 6YTK |
| Calcium homeostasis modulator (CALHM5) in the presence of EDTA (expressed in HEK293 cells) | Homo sapiens | Cryo-EM single particle analysis | 2.80 Å | 7D61 |
| Calcium homeostasis modulator (CALHM6), decameric in presence of Ca2+ (expressed in HEK293 cells) | Homo sapiens | Cryo-EM single particle analysis | 4.39 Å | 6YTV |
Table 1. Structural research of gap junctions and related channels.
At Creative Biostructure, we are at the forefront of providing cutting-edge structural analysis services, such as Cryo-EM studies and molecular dynamics simulations. Our team of highly skilled experts and state-of-the-art facilities enable us to unravel the intricate structures of biological macromolecules and their complexes. With our advanced techniques and methodologies, we can assist researchers in gaining comprehensive insights into the structure and function of connexin channels and gap junctions. By understanding these molecular architectures, we can contribute to the development of novel therapeutic strategies targeting intercellular communication and associated diseases.
Contact us to leverage our expertise and unlock the secrets of gap junctions and related channels. Together, we can push the boundaries of structural biology and pave the way for innovative discoveries and therapeutic interventions.
References
- Choi W, et al. The structures and gating mechanism of human calcium homeostasis modulator 2. Nature. 2019, 576(7785): 163-167.
- Flores J A, et al. Connexin-46/50 in a dynamic lipid environment resolved by CryoEM at 1.9 Å. Nature Communications. 2020, 11(1): 4331.
- Drożdżyk K, et al. Cryo-EM structures and functional properties of CALHM channels of the human placenta. Elife. 2020, 9: e55853.