Structural Research of Flaviviridae
The Flaviviridae are a group of positive single-stranded RNA viruses that mostly infect mammals and birds. The genetic material is single-stranded linear RNA, and the viral particles have an outer coat with a diameter of about 40-60nm. The flavivirus genus of this family, which includes several major human pathogens, such as dengue virus (DENV), Zika virus (ZIKV), and Japanese encephalitis virus (JEV), poses a major threat to human health. In recent years, to better understand the infection mechanism of Flaviviridae family members and to discover new antiviral strategies, researchers have determined the structure of Flavivirus particles by cryo-electron microscopy (cryo-EM) at high resolution, which provides a promising starting point for the further development of Flavivirus vaccines or therapeutics.
 Figure 1. High-resolution structure determination of flavivirus particles using cryo-EM. (Hardy JM, et al., 2021)
Figure 1. High-resolution structure determination of flavivirus particles using cryo-EM. (Hardy JM, et al., 2021)
The Overall Structure of Flaviviridae Members
The viral particles of flaviviruses are usually spherical with a lipid envelope. The RNA genome encodes a long open reading region (ORF) flanked by capped 5-termini with methylated nucleotides. In cooperation with viral and cellular proteases, the translated polypeptide is cleaved into a capsid protein (C protein), a pro-membrane protein (prM protein), an envelope protein (E protein), and seven nonstructural (NS) proteins. Of these, the C proteins are essential for the assembly and maturation of viral particles and are the most promising drug target candidates. The NS proteins are proteases primarily involved in the cleavage of polyproteins and the regulation of host cell responses. The NS2A and NS3 are also involved in viral particle assembly through direct interaction with C proteins. The NS1 regulates the production of infectious particles.
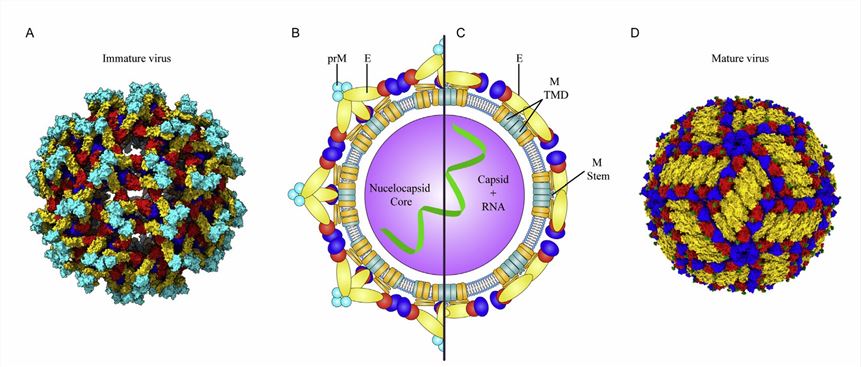 Figure 2. Structure of flavivirus particles. (Nicholls CMR, et al., 2020)
Figure 2. Structure of flavivirus particles. (Nicholls CMR, et al., 2020)
Structural Analysis of the Flavivirus C Protein
The C protein plays an essential role in the host cell and viral life cycle. It protects viral RNA and interacts with various host proteins to promote viral proliferation. Therefore, research on the structure of the C protein and its action mechanism with host nuclear proteins may provide several ideal targets for vaccine development. The structure of the C protein has been resolved by NMR spectroscopy and X-ray crystallography. The structures show that the C proteins are dimers in a double α-helix conformation, including four α-helices connected by short loops, with the N termini of the dimer facing each other to form a tetramer with a positively charged surface. Each C protein molecule contains a hydrophobic region and a highly cationic region.
Creative Biostructure specializes in the structural research of viruses. We offer a wide range of carefully characterized virus-like particles (VLPs) products that can be used as valuable tools for research into virus entry, immunology, and vaccine development. Our VLP products closely mimic the morphology and surface characteristics of target viruses, providing a safe and non-infectious alternative for in-depth research.
| Cat No. | Product Name | Virus Name | Source | Composition |
| CBS-V067 | Dengue Virus Serotype 1 VLP (E, M, preM Proteins) | Dengue virus | Mammalian cell recombinant | E, prM; M |
| CBS-V567 | DENV 2 (16681) VLP (prM; E Proteins) | Dengue virus | Mammalian cell recombinant | prM; E |
| CBS-V605 | HCV genotype 1b VLP (core; E1; E2 Proteins) | Hepatitis C virus | Mammalian cell recombinant | Core; E1; E2 |
| CBS-V664 | Japanese encephalitis virus VLP (prM; M; E Proteins) | Japanese encephalitis virus | Insect cell recombinant | prM; M; E |
| Explore All Flaviviridae Virus-like Particle Products | ||||
With deep expertise in virology and cutting-edge technologies, Creative Biostructure provides comprehensive viral structure analysis solutions to enhance clients' understanding of viral structural biology and support a wide range of applications in research and development. Our highly skilled scientists are proficient in Cryo-EM technology and are committed to providing accurate and detailed structural analyses of viral particles and virus-like particles, revealing complex structures and molecular interactions.
In addition, we offer a range of high-quality virus-like particles (VLPs) products that can be used for antiviral drugs and vaccine development. If we do not have the VLP product you require in our current catalog, please feel free to contact us. We believe that by harnessing the power of viral structure analysis and providing innovative VLP products, we will help you achieve breakthroughs in viral research, drug discovery, and diagnostics.
References
- Hardy JM, et al. A unified route for flavivirus structures uncovers essential pocket factors conserved across pathogenic viruses. Nat Commun. 2021. 12(1): 3266.
- Nicholls CMR, et al. Structure-guided paradigm shifts in flavivirus assembly and maturation mechanisms. Adv Virus Res. 2020. 108: 33-83.
- Zhang X, et al. Structure and function of capsid protein in flavivirus infection and its applications in the development of vaccines and therapeutics. Vet Res. 2021. 52(1): 98.
- Zhao R, et al. Flavivirus: From Structure to Therapeutics Development.Life (Basel). 2021. 11(7): 615.