Structural Research of Vacuolar Iron Transporter (VIT) Family
To avoid cellular damage caused by excess free iron, plants, yeast, and protists maintain iron homeostasis by transporting iron into membrane-bound vesicles through the action of H+-dependent reverse transporter proteins (known as Ccc1 in yeast and the vesicular iron transporter protein VIT in plants). It is found that VIT1 is essential for iron homeostasis in plants, and overexpression of VIT1 increased iron accumulation in crops, which may have applications in the treatment of human iron deficiency diseases. In addition, VIT1 homologs of Plasmodium falciparum have been suggested as potential drug targets for the treatment of malaria. Therefore, it is essential to understand the structure and molecular mechanism of VIT.
Advances in research on the structure and function of VIT1/Ccc1 family
Recently, researchers have resolved the crystal structure of the Eucalyptus grandis EgVIT1 protein, which is essential for analyzing the iron transport mechanism. VIT1 possesses five transmembrane structural domains (TMD1-5) and a cytoplasmic loop containing three α-helices (H1-3) between TMD2 and TMD3. The interface between the two protomers of the VIT1 dimer constitutes a channel for the translocation of metal ions. In this cavity, TMD1 and TMD2 provide tyrosine, methionine, and carboxylation groups that confer a hydrophilic environment suitable for metal ion translocation. Other TM fragments are arranged around this cavity. To transport iron to the vesicle, the glycine and proline residues undergo a kinking motion, which allows iron to enter the vesicle. Research has shown that the highly conserved glycine residue within the second TMD is essential for VIT1 iron transport activity.
Analyzing the overall structure of PfVIT
P. falciparum, the most virulent malaria parasite in humans, has a homolog of plant VIT called PfVIT. Researchers constructed a homology model of the PfVIT protein using the x-ray crystal structure of plant VIT1 as a template. The structure shows that PfVIT is a homodimer, with each subunit containing a transmembrane structural domain (TMD) and a cytoplasmic metal-binding structural domain (MBD). Each PfVIT protomer consists of a bundle of five transmembrane (TM) helices, with the N- and C-termini located in the cytoplasmic and luminal parts of the membrane, respectively. Viewed from the luminal side of the protein perpendicular to the membrane plane, TM2-5 of each protomer is arranged counterclockwise around the center TM1. TM2 extends into the cytoplasm and is connected to three short helices (H1-H3). At the dimer interface between the TMDs is a large funnel-shaped cavity into which solvents from the cytoplasm can enter.
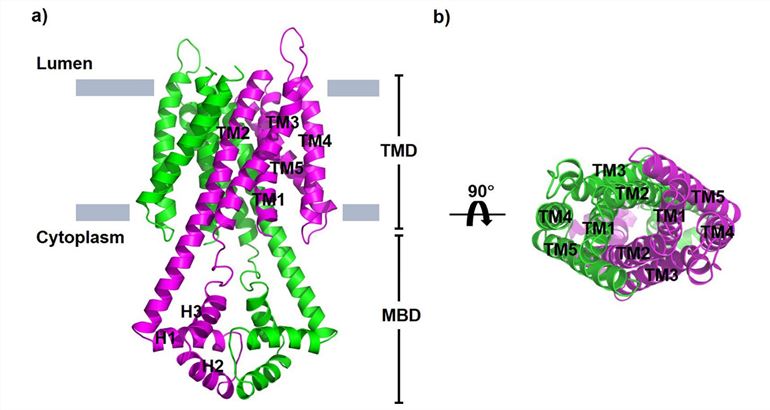 Figure 1. Structural model of the P. falciparum vacuolar iron transporter PfVIT. (Sharma P, et al., 2021)
Figure 1. Structural model of the P. falciparum vacuolar iron transporter PfVIT. (Sharma P, et al., 2021)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| Iron transporter VIT1 with zinc ions | Eucalyptus grandis | X-ray diffraction | 2.7 Å | 6IU3 |
| Iron transporter VIT1 with cobalt ion | Eucalyptus grandis | X-ray diffraction | 3.5 Å | 6IU4 |
| Cytoplasmic metal binding domain with iron ions | Eucalyptus grandis | X-ray diffraction | 3 Å | 6IU9 |
Table 1. Structural research of the vacuolar iron transporter (VIT) family.
Creative Biostructure invites researchers and pharmaceutical companies to partner with us and utilize our outstanding structural analysis services. With extensive industry experience, we have pioneered innovative methods and cutting-edge technologies for structural analysis of vacuolar iron transporter (VIT) family proteins.
Our team of expert scientists employs advanced techniques such as X-ray crystallography, cryo-electron microscopy (cryo-EM), and NMR spectroscopy to obtain high-resolution structural data, providing valuable insights into the structure and function of the VIT family. If you are interested in our services, please contact us to learn how our state-of-the-art capabilities can advance your scientific goals.
References
- Sharma P, et al. Characterization of the substrate binding site of an iron detoxifying membrane transporter from Plasmodium falciparum. Malar J. 2021. 20(1): 295.
- Sorribes-Dauden R, et al. Structure and function of the vacuolar Ccc1/VIT1 family of iron transporters and its regulation in fungi. Comput Struct Biotechnol J. 2020. 18: 3712-3722.
- Kato T, et al. Crystal structure of plant vacuolar iron transporter VIT1. Nat Plants. 2019. 5(3): 308-315.