MemPro™ Microsomal prostaglandin E synthase
Creative Biostructure provides custom MemPro™ gene-to-structure services for Microsomal prostaglandin E synthase.
Prostaglandin E synthase (PGESs), also known as terminal synthases, is a member of MAPEG (membrane associated proteins in eicosanoid and glutathione metabolism) superfamily that catalyze the biosynthesis of active PGE2 from cyclooxygenases (COX-1- and COX-2) derived PGH2.
Prostaglandins (PGs) are a class of potent biological lipid mediators, which are found ubiquitously in almost all mammalian tissues, and mediate diverse physiological and pathological processes. The biological functions of PGE2 are primarily mediated through its specific binding with one or more of its four receptors EP1–4. PGE2 acts as a predominant mediator in various inflammatory disease conditions, such as, rheumatoid arthritis, and the inhibition of its synthesis by nonsteroidal anti-inflammatory agents (NSAIDs) has been viewed as an important anti-inflammatory therapy since long time.
Three types of PGESs have been discovered so far, which are referred to as microsomal PGES-1 (mPGES-1), microsomal PGES-2 (mPGES-2), and cytosolic PGES (cPGES). Among them, cPGES and mPGES-2 are expressed constitutively, while mPGES-1 is an inducible enzyme. The cell expression studies demonstrate that cPGES is coupled with COX-1, mPGES-2 with both COX-1 and COX-2, whereas mPGES-1 is specifically coupled with COX-2. The expression of mPGES-1 is induced by various inflammatory stimuli, such as interleukin-1-beta (IL-1β) and is the major source of inducible PGE2. The induction of mPGES-1 by inflammatory stimuli and its role in the production of COX-2- derived PGE2 makes it an alternative target to COX-2 for the management of inflammatory conditions.
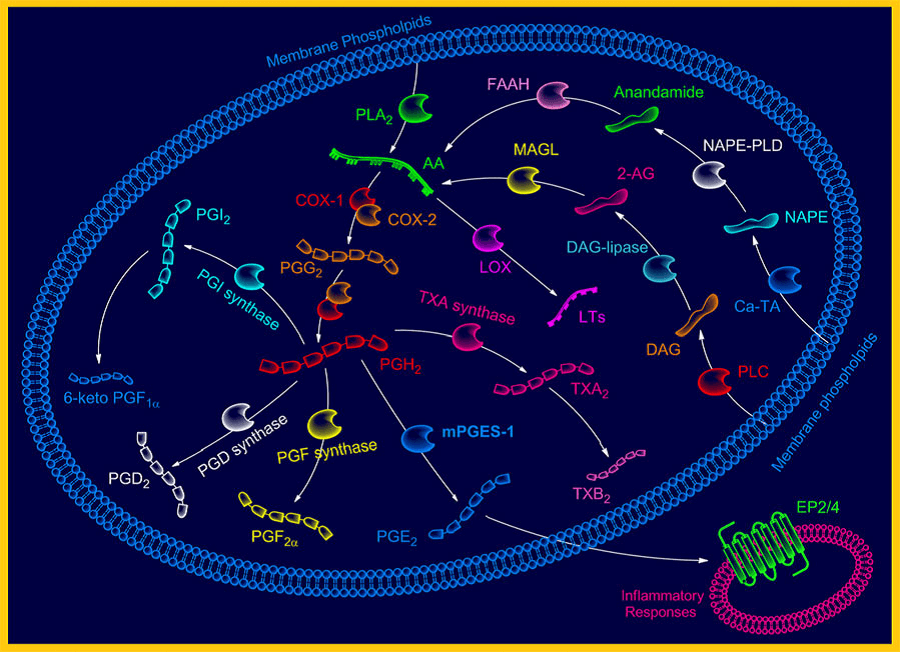 Figure 1. Role of mPGES-1 in arachidonic acid cascade
Figure 1. Role of mPGES-1 in arachidonic acid cascade
Electron crystallography structure of mPGES-1 has showed present distinct structure domain with LTC4 synthase and FLAP, but shared similar characters with microsomal Glutathione S transferase (MGST1). mPGES-1 overall fold consist of a four-helix bundle that packs together to form a homotrimer. The most prominent differentiating feature of the mPGES-1 structure with other MAPEG members is the insert between TMI and TMII that folds into a small well-structured domain (the C-domain) that forms part of the active site cavity.
Since COX-1 regulates Gastrointestinal (GI), renal, and vascular functions, to overcome the side effects of cNSAIDs, the selective inhibition of inducible COX-2 was viewed as a better alternative, which in turn lead to the development of coxibs. Although existing mPGES-1 inhibitors display good in vitro activity, they describe a poor pharmacokinetics profile due to strong serum binding and poor bioavailability. Therefore, licofelone, an anti-inflammatory drug molecule reported to exert biological action through the inhibition of PG and leukotriene biosynthesis pathway, has demonstrated the beneficial potential in phase-III clinical trials results for osteoarthritis. licofelone described excellent gastrointestinal tolerability and also inhibit COX-1 COX-2 and PEGS-1 at extremely low IC50 value.
References:
Singh Bahia M, Kumar Katare Y, Silakari O, et al. Inhibitors of Microsomal Prostaglandin E2 Synthase‐1 Enzyme as Emerging Anti‐Inflammatory Candidates[J]. Medicinal research reviews, 2014, 34(4): 825-855.
Sjögren T, Nord J, Ek M, et al. Crystal structure of microsomal prostaglandin E2 synthase provides insight into diversity in the MAPEG superfamily[J]. Proceedings of the National Academy of Sciences, 2013, 110(10): 3806-3811.
