Atherosclerosis and Myocardial Injury Exosome Services
Cardiovascular Disease (CVD) remains the leading cause of death globally. The pathology spans from the chronic inflammation of Atherosclerosis (plaque formation) to the acute catastrophe of Myocardial Infarction (MI). Exosomes participate in every step: damaged endothelial cells release vesicles that trigger plaque buildup, while stem cell-derived exosomes offer a promising therapy to reduce fibrosis and regenerate heart tissue after a heart attack.
We provide a holistic Cardiovascular Exosome Research Solution. Whether you are investigating the role of Macrophage Exosomes in foam cell formation, validating MSC-derived Exosomes for myocardial repair, or identifying circulating biomarkers for Plaque Instability, our platform integrates specialized isolation techniques, cellular disease models, and physiological animal imaging to accelerate your cardiovascular discoveries.
Critical Frontiers in Atherosclerosis and Myocardial Injury
Research in this field is pivoting from simple lipid management to targeting the inflammatory mechanisms of plaque rupture and the regenerative limits of the heart.
- Plaque Instability & Rupture: The primary clinical danger is not plaque size, but stability. Understanding how exosomes mediate the degradation of the fibrous cap (via MMPs) and drive inflammation within the vulnerable plaque is a top priority for preventing acute events.
- Barriers to Myocardial Regeneration: Adult cardiomyocytes have negligible regenerative capacity. Following a heart attack (MI), the heart scars rather than heals. The central challenge is identifying exosomal signals (e.g., from stem cells or neonates) that can re-activate the cardiomyocyte cell cycle.
- Ischemia-Reperfusion Injury (IRI): Restoring blood flow after MI is necessary but paradoxically causes significant cellular damage. Deciphering the exosomal crosstalk between endothelial cells and cardiomyocytes during reperfusion is key to developing cardioprotective strategies.
- Resolution of Inflammation: Both atherosclerosis and MI repair rely on the timely switch of macrophages from a pro-inflammatory (M1) to a reparative (M2) phenotype. Research is focused on how exosomes regulate this immune transition to prevent chronic failure.
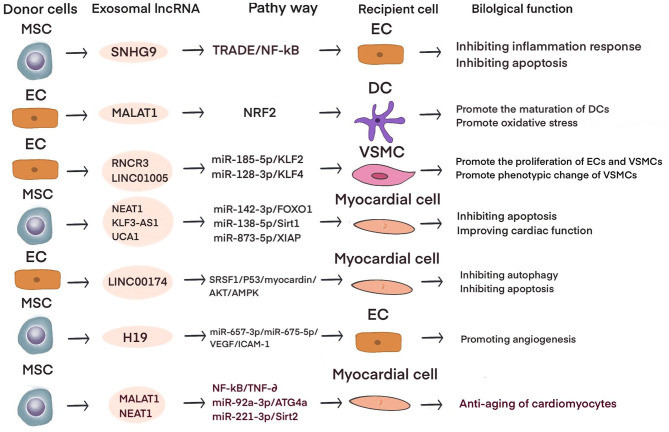 Figure 1. Exosomal lncRNA's role in cardiovascular disease preclinical studies. (Yuan Z, et al., 2021)
Figure 1. Exosomal lncRNA's role in cardiovascular disease preclinical studies. (Yuan Z, et al., 2021)
Comprehensive Service Portfolio for CVD
We offer an integrated matrix of services tailored to your specific cardiovascular model, covering biomarkers, mechanism, and therapy.
| Research Focus | Our Specialized Approach & Solution | Key Services Applied |
|---|---|---|
| Atherosclerosis (Mechanism) | Foam Cell & Inflammation Models: We model atherogenesis in vitro by treating macrophages with ox-LDL. We test if exosomes inhibit lipid uptake (Oil Red O staining) or pro-inflammatory cytokine release. | Immunomodulation and Inflammation Assays |
| Myocardial Injury (Therapy) | MSC Exosome Manufacturing: We produce MSC-derived exosomes (Bone Marrow/Umbilical Cord) and validate their cardioprotective effects in hypoxia-injured cardiomyocytes. | MSC and Stem Cell Derived Exosome Therapy |
| Tissue Profiling (Discovery) | Plaque/Heart Tissue Isolation: Circulating exosomes are diluted. We use specialized Tissue Dissociation protocols to isolate exosomes directly from aortic plaques or fibrotic heart tissue for proteomic analysis. | Animal Tissue Exosome Isolation |
| In Vivo Efficacy (Animal Models) | LAD Ligation & ApoE Models: We perform LAD Ligation (for MI) or use ApoE-/- mice (for Atherosclerosis). We evaluate cardiac function via Echocardiography and plaque size via Histology. | In Vivo Exosome Functional Assays |
Core Technologies for Cardiovascular Research
We highlight specialized technologies that address the physiological complexity of the cardiovascular system.
Foam Cell Formation Assay
Modeling Atherogenesis: The hallmark of atherosclerosis is the transformation of macrophages into cholesterol-laden foam cells. We utilize an ox-LDL induced Macrophage Model. Researchers can treat these cells with therapeutic exosomes (e.g., from M2 macrophages) and quantify the reduction in lipid accumulation using Oil Red O staining and cholesterol uptake assays, providing a direct readout of anti-atherosclerotic potential.
LAD Ligation & Echocardiography
The Gold Standard for MI: To test cardiac repair therapies, we perform Left Anterior Descending (LAD) Artery Ligation in mice/rats to induce myocardial infarction. Post-treatment, we utilize high-resolution small animal Echocardiography (M-mode/B-mode) to non-invasively measure Ejection Fraction (EF) and Fractional Shortening (FS), ensuring clinically relevant efficacy data.
Heart/Aorta Tissue Exosome Isolation
Source Matters: While plasma is useful, the pathology happens in the tissue. We offer specialized Animal Tissue Exosome Isolation protocols optimized for the fibrous matrix of the aorta and the myocardium. This allows for the specific profiling of vesicles trapped within the plaque or the infarct zone, revealing local signaling networks often missed in blood.
Application Spotlight: Epigenetic Engineering Enhances Cardiac Repair
This analysis highlights how genetically engineering stem cell exosomes to carry specific epigenetic regulators can significantly boost their therapeutic potency for myocardial injury.
Featured Technologies:
- Genetically Engineered Exosome Production
- Myocardial Infarction (LAD) Animal Models
Literature Interpretation:
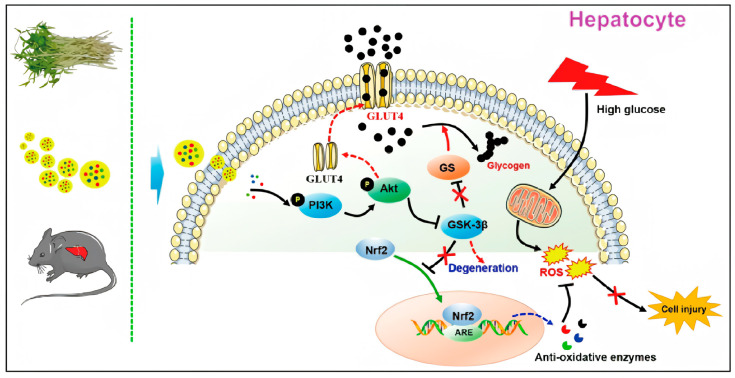
Adipose-derived stem cell (ADSC) exosomes show promise for treating myocardial infarction, but their basal efficacy is often limited. Researchers sought to enhance this by genetically engineering ADSCs to overexpress Sirt6, a key epigenetic regulator (histone deacetylase). The resulting "Sirt6-enriched exosomes" were isolated and tested in a mouse model of Myocardial Ischemia-Reperfusion Injury (I/R). The study demonstrated that these engineered exosomes were far superior to native exosomes in reducing infarct size and preserving cardiac function. Mechanistically, they mitigated oxidative stress and inflammation by modulating the Nrf2 signaling pathway via epigenetic silencing. This study validates the power of "next-generation" engineered exosomes and underscores our capabilities in genetic modification and in vivo cardiac efficacy testing.
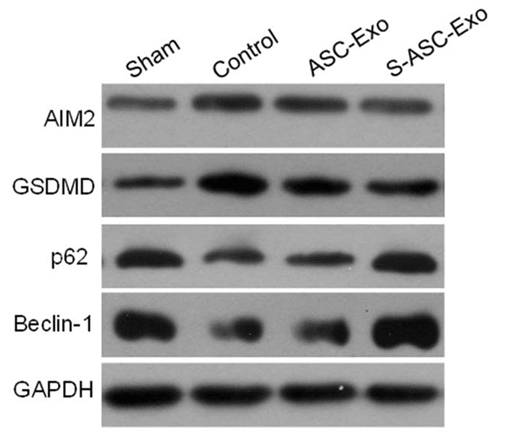 Figure 2. Western blot analysis of AIM2, GSDMD, p62, and Beclin-1 protein expressions in H9c2 cells under AR treatment and S-ASC-Exo influence. (Liu K, et al., 2024)
Figure 2. Western blot analysis of AIM2, GSDMD, p62, and Beclin-1 protein expressions in H9c2 cells under AR treatment and S-ASC-Exo influence. (Liu K, et al., 2024)
Start Your Cardiovascular Research Project
Leverage our comprehensive platform to accelerate your discovery, from plaque biology to heart repair.
How It Works: Our Project Pathway
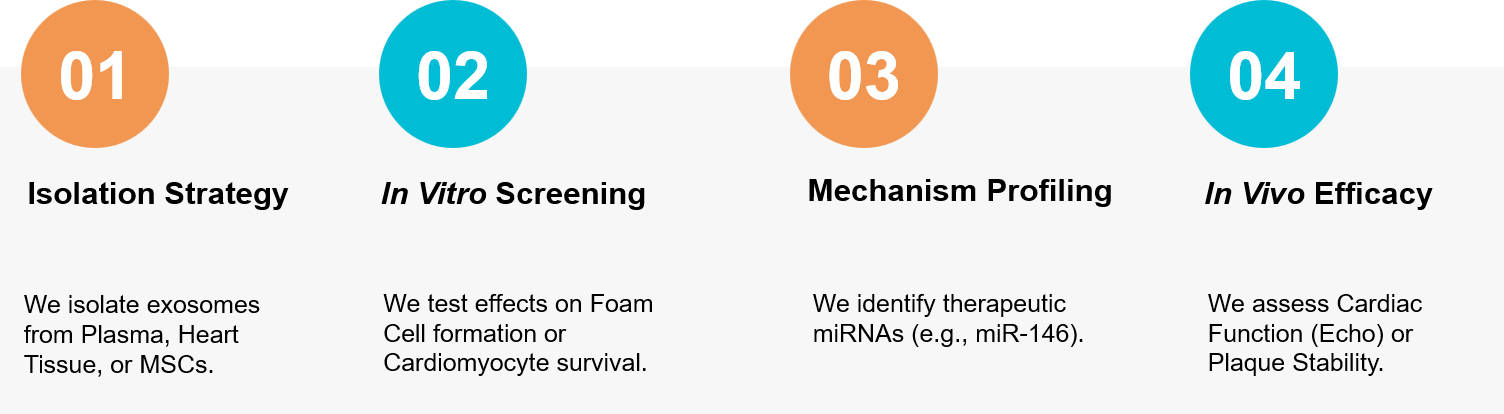 Figure 3. Workflow for isolating cardiovascular exosomes, screening mechanisms, and validating therapy in Atherosclerosis and MI models. (Creative Biostructure)
Figure 3. Workflow for isolating cardiovascular exosomes, screening mechanisms, and validating therapy in Atherosclerosis and MI models. (Creative Biostructure)
Ready to advance your research on Heart Failure or Atherosclerosis? Our cardiovascular experts are available to build a custom study plan tailored to your needs. Contact us today to discuss your project.
References
- Yuan Z, Huang W. New Developments in Exosomal lncRNAs in Cardiovascular Diseases. Front Cardiovasc Med. 2021 Jul 8;8:709169.
- Liu K, Wang H, Wang Y, et al. Exploring the therapeutic potential of Sirt6-enriched adipose stem cell-derived exosomes in myocardial ischemia-reperfusion injury: unfolding new epigenetic frontiers. Clin Epigenetics. 2024 Jan 3;16(1):7.