Cryo-Electron Microscopy (Cryo-EM)-Based Exosome Characterization Service
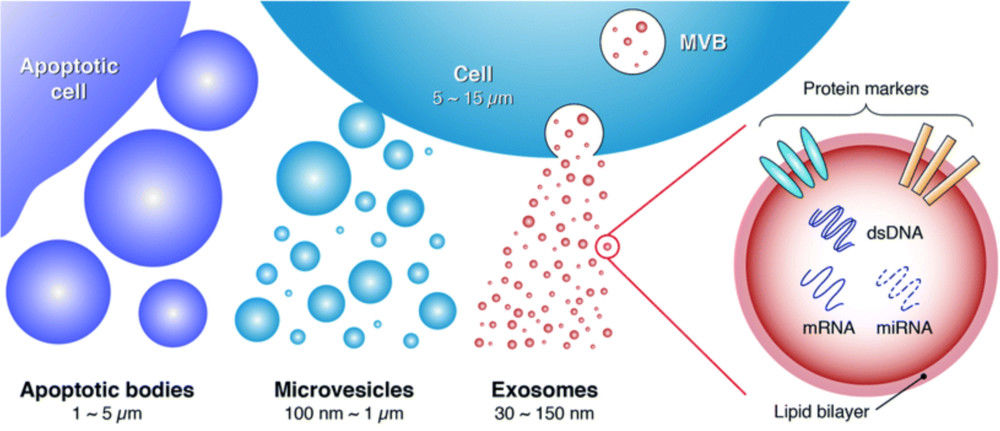
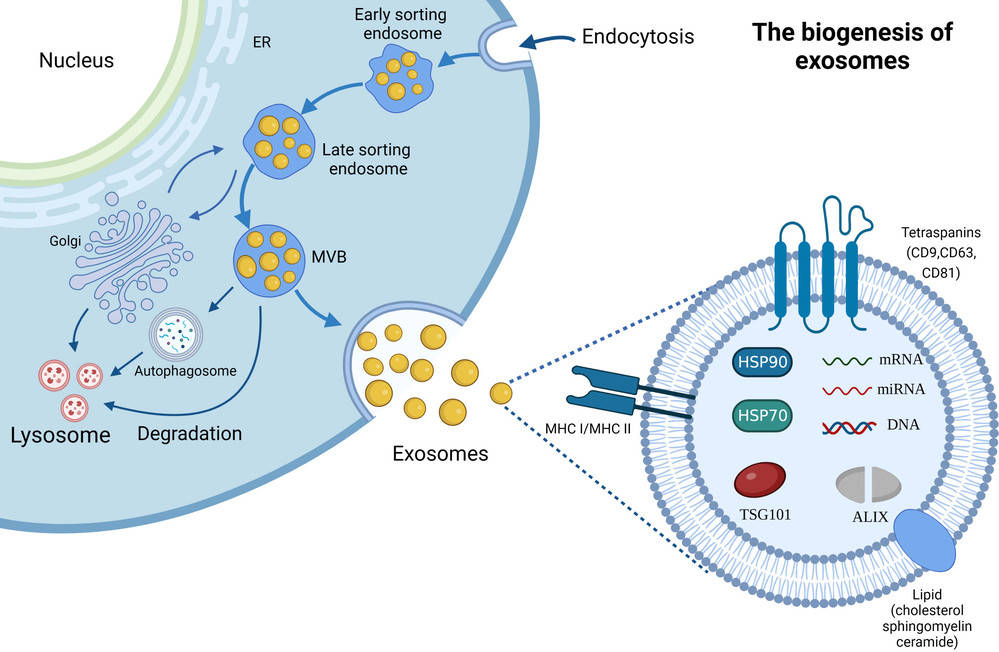
Extracellular vesicles (EVs), including exosomes, are nanoscale lipid bilayer vesicles involved in intercellular communication, disease progression, and therapeutic delivery. Accurate structural analysis is essential for understanding their biological functions and supporting translational applications. Cryo-electron microscopy (Cryo-EM) provides unparalleled resolution for exosome characterization, enabling visualization of vesicles in their near-native state without the need for chemical fixation or staining.
At Creative Biostructure, we deliver state-of-the-art Cryo-EM exosome characterization services that comply with international standards, including the MISEV2023 guidelines, ensuring reproducible, publication-ready data for both academic and industrial clients.
Why Use Cryo-EM for Exosomes?
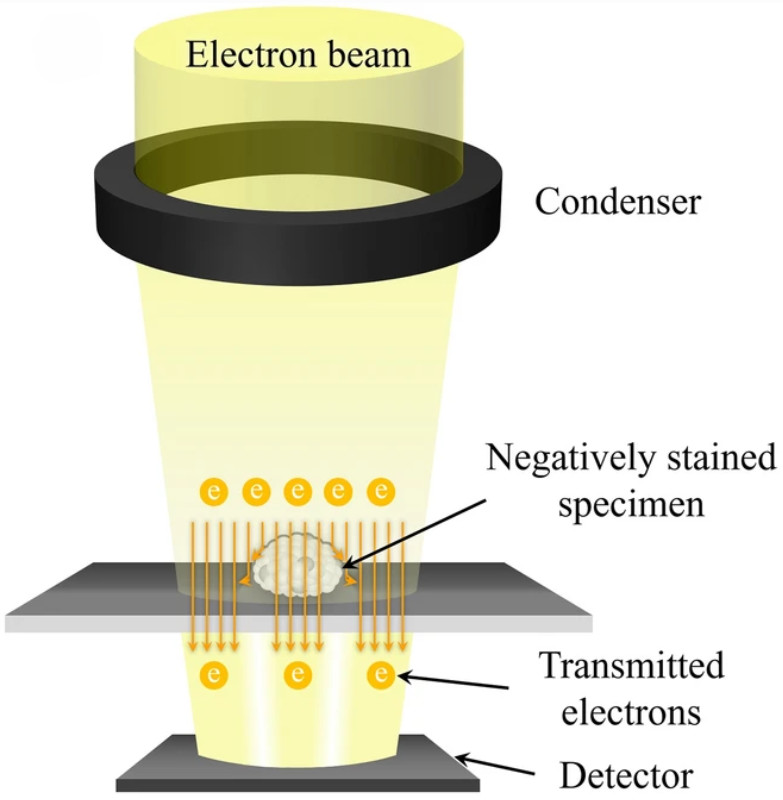
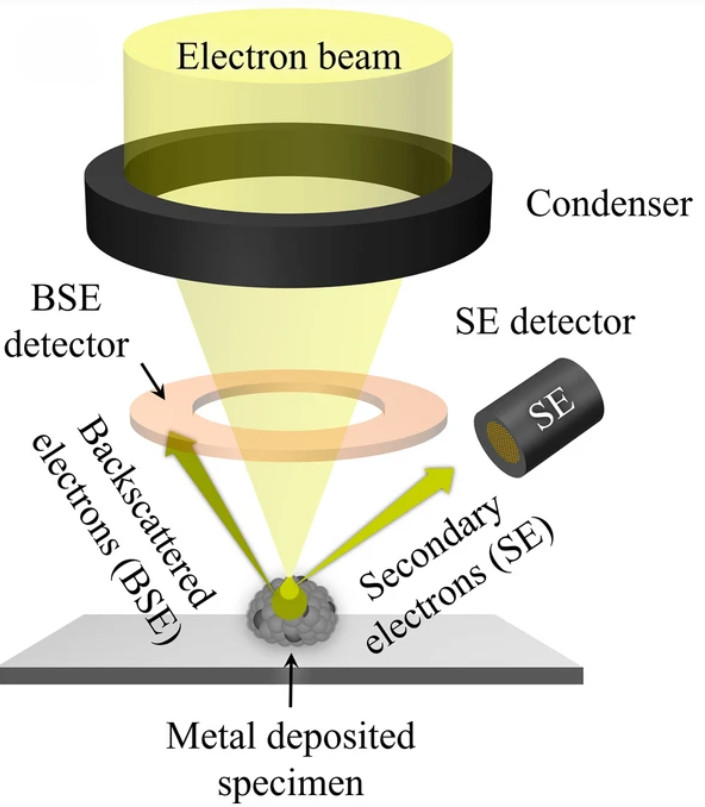
Traditional electron microscopy techniques such as transmission electron microscopy (TEM) and scanning electron microscopy (SEM) are valuable but introduce artifacts due to dehydration, fixation, or metal coating. In contrast, Cryo-EM preserves vesicles in a vitrified, hydrated state, maintaining their native spherical morphology and structural integrity.
Key advantages include:
- Natural morphology preservation: eliminates deformation caused by dehydration.
- Visualization of lipid bilayers: allowing clear distinction between vesicles and impurities.
- High resolution (~2 nm): enabling detailed imaging of nanoscale structures.
- Quantitative imaging: all particles within a given field are observed, not only those adhered to a surface.
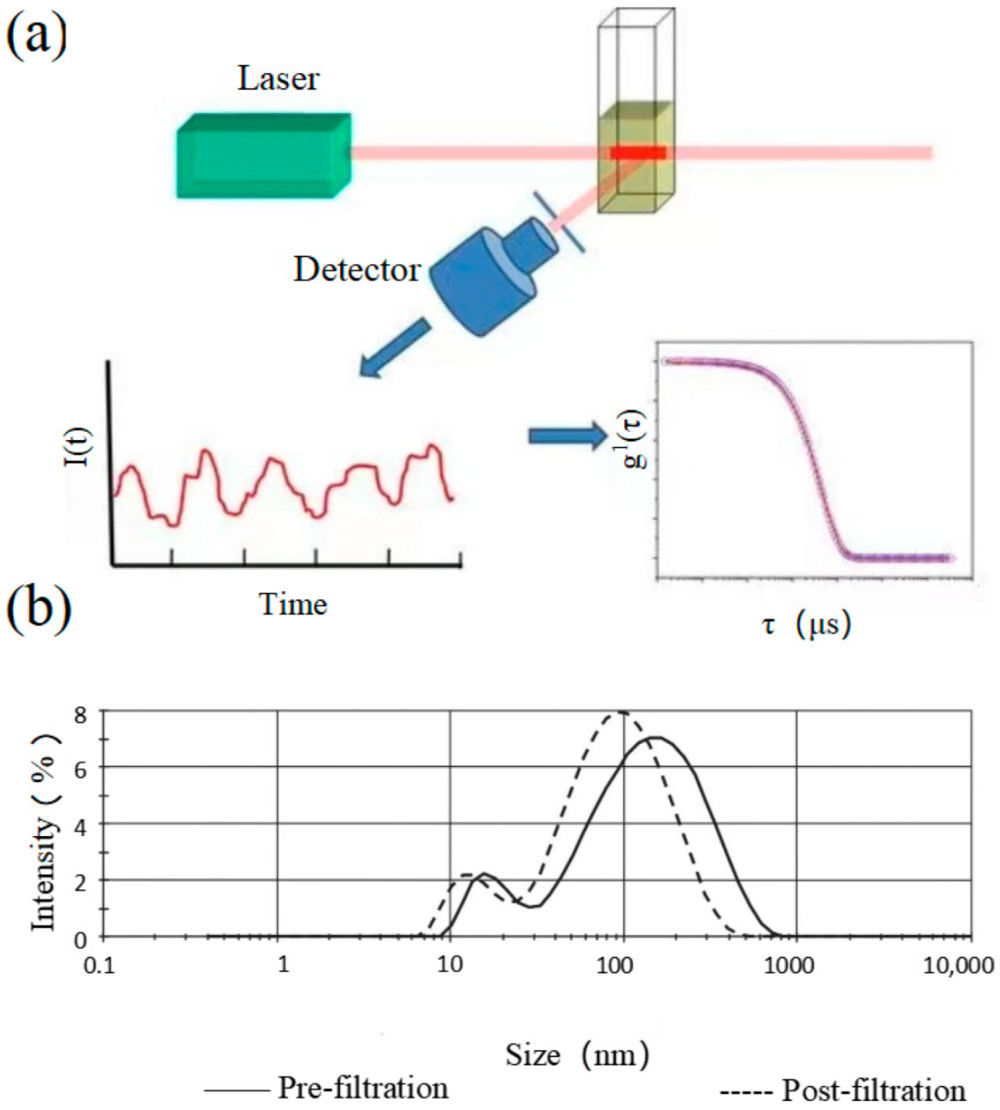
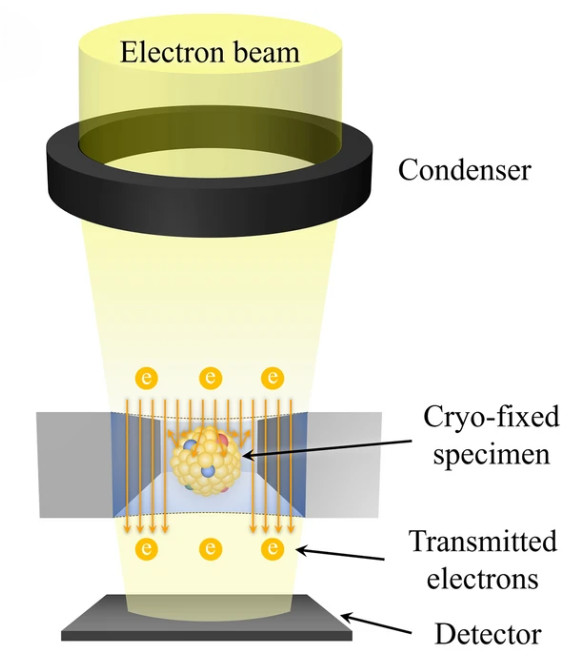 Figure 1. Principle of Cryo-Transmission Electron Microscopy (Cryo-TEM) for EVs. (Kwon Y, et al., 2022)
Figure 1. Principle of Cryo-Transmission Electron Microscopy (Cryo-TEM) for EVs. (Kwon Y, et al., 2022)
Cryo-EM vs. Other Electron Microscopy Techniques
Choosing the right imaging approach is critical for reliable exosome analysis.
| Feature | SEM | TEM | Cryo-EM (Cryo-TEM) |
|---|---|---|---|
| Sample Preparation | Metal coating required | Fixation & negative stain | Rapid freezing, vitrification |
| Morphology Accuracy | External surface only | Cup-shaped artifacts | Preserves natural spherical form |
| Resolution | ~10 nm | ~1-2 nm | ~2 nm |
| Lipid Bilayer Visibility | Limited | Partial with staining | Clearly resolved |
| Quantitative Capability | Low | Moderate | High, all particles imaged |
This comparison highlights why Cryo-EM is the method of choice for exosome characterization when precision and structural fidelity are required.
Our Cryo-EM Exosome Characterization Workflow
Our workflow covers the full process from project consultation to data delivery, ensuring accurate, reproducible, and publication-ready results:
Consultation & Study Design
We begin with a detailed discussion to define research objectives, sample requirements, and analytical parameters, tailoring the workflow to each project.
Sample Submission & Preparation
Clients may submit either purified exosome/EV samples under recommended storage and transport conditions or raw biological materials, from which our team can perform standardized isolation and purification. Pre-analysis quality assessment (particle concentration, buffer compatibility, and potential contaminants) to ensure Cryo-EM readiness.
Grid Preparation & Vitrification
EV samples are applied onto EM grids in small droplets and rapidly plunge-frozen in liquid ethane, preserving them in a vitrified, hydrated state without deformation; multiple grids are prepared in parallel to ensure representative sampling.
High-Resolution Cryo-EM Imaging
Imaging performed at multiple ice thicknesses and grid regions to capture vesicle diversity. Both low- and high-magnification images are acquired for global quality assessment and detailed structural evaluation.
Particle Analysis & Quantification
Each project involves the analysis of hundreds to thousands of vesicles, assessing key parameters such as morphology, size distribution, lamellarity, encapsulation efficiency, and surface features, with optional immunogold labeling available for targeted surface marker visualization.
Data Processing & Validation
Our data processing includes rigorous image selection, noise reduction, and structural annotation, followed by cross-checking against MISEV2023 reporting guidelines to ensure standardized results, with optional validation of batch-to-batch reproducibility or stability when required.
Report Generation & Delivery
A comprehensive report is provided, including representative Cryo-EM images, statistical analyses, and expert interpretation tailored to the client's research goals.
Our scientists remain available for result discussion, follow-up consultation, and recommendations for next-step experiments or complementary assays.
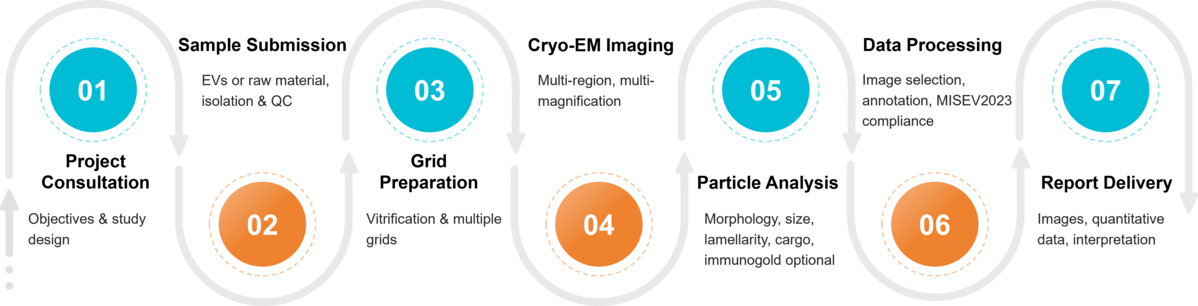 Figure 2. Project Workflow for Exosome Characterization by Cryo-EM. (Creative Biostructure)
Figure 2. Project Workflow for Exosome Characterization by Cryo-EM. (Creative Biostructure)
Sample Requirements for Cryo-TEM Analysis
To ensure high-quality imaging and reproducible results, samples must meet certain requirements. Creative Biostructure accepts both purified exosome/EV samples and raw materials for in-house isolation and preparation.
| Category | Requirement |
|---|---|
| Sample Type | Purified exosomes/EVs or raw biological material (e.g., cell culture supernatant, body fluids) |
| Minimum Volume | ~50-100 µL purified EVs, or ≥1 mL raw material for isolation and preparation |
| Concentration | 1 × 108 – 1 × 1010 particles/mL (recommended for purified EVs) |
| Buffer Conditions | PBS or cryo-compatible buffer; avoid high salt or detergent-containing buffers |
| Storage & Transport | Keep on ice or at 4 °C for short-term; ship on dry ice for long-distance transport |
| Optional Services | Exosome isolation and purification, pre-analysis quality check (NTA, protein/RNA assay) |
What Deliverables Will You Receive
- Raw and processed Cryo-EM images
- Size distribution and morphology data
- Lamellarity and membrane integrity analysis
- Encapsulation and surface feature assessment
- Representative micrographs with statistical summaries
- Comprehensive technical report with interpretation
- Optional validation results (stability, batch comparison, immunogold labeling)
Applications of Cryo-EM in Exosome Research
Our Cryo-EM platform enables researchers to obtain both qualitative and quantitative insights into exosome preparations, supporting a wide range of applications:
- Morphology & Size Distribution: distinguish vesicle populations with nanometer resolution.
- Membrane Integrity & Lamellarity: evaluate bilayer structure and vesicle quality.
- Cargo Encapsulation: assess internal payloads and encapsulation efficiency.
- Surface Marker Localization: optional immunogold labeling for protein-specific analysis.
- Stability & Batch Comparison: monitor vesicle integrity across manufacturing processes or storage conditions.
Why Choose Creative Biostructure?
- Expertise: Our team has extensive experience in exosome biology and advanced EM imaging.
- Cutting-edge instrumentation: High-end Cryo-EM platforms optimized for vesicle analysis.
- Tailored services: Customizable workflows for both academic and industrial needs.
- Confidentiality & Trust: Strict data security and project-specific agreements.
Case Study
Case: Cryo-EM Characterization of Porcine Seminal Extracellular Vesicles
Background
Seminal plasma (SP) is enriched in extracellular vesicles, yet their heterogeneity is poorly understood. This study applied cryo-EM to characterize porcine seminal EVs (sEVs) and associate vesicle subtypes with tissue of origin.
Methods
sEVs were isolated from 21 ejaculates (whole and fractionated). Purification used centrifugation, ultrafiltration, and size exclusion chromatography. A total of 1,840 cryo-EM images were analyzed for morphology, size, electron density, and coronal layers, complemented by flow cytometry validation.
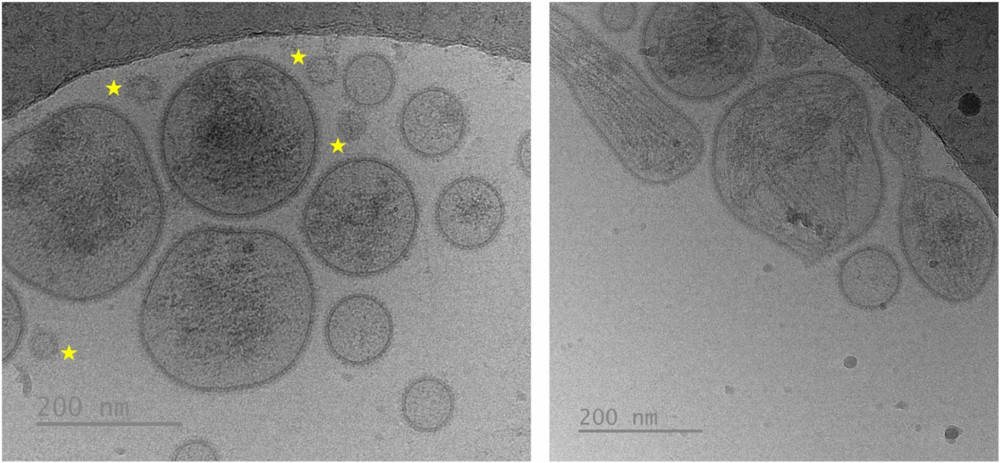 Figure 3. Cryo-EM Images of Seminal Extracellular Vesicles (sEVs). Vesicles exhibit heterogeneity in morphology and size. The yellow star highlights non-vesicular extracellular particles, which resemble small sEVs in size but lack a surrounding membrane, distinguishing them from true vesicles. (Parra A, et al., 2024)
Figure 3. Cryo-EM Images of Seminal Extracellular Vesicles (sEVs). Vesicles exhibit heterogeneity in morphology and size. The yellow star highlights non-vesicular extracellular particles, which resemble small sEVs in size but lack a surrounding membrane, distinguishing them from true vesicles. (Parra A, et al., 2024)
Results
Most vesicles (83.1%) were small (<200 nm), rounded, and often carried a peripheral corona (43.1%), while larger vesicles (16.9%) were irregular, more electron-dense, and less corona-positive (15.1%). Cargo was visible in 93% of large sEVs versus ~30% of small ones. Small sEVs predominated in epididymal/prostatic fractions, while large vesicles were enriched in seminal vesicle fractions.
Conclusion
Cryo-EM revealed distinct morphological subtypes of porcine sEVs, indicating organ-specific origins and emphasizing cryo-EM's value in defining EV heterogeneity.
At Creative Biostructure, we combine advanced Cryo-EM technology with extensive expertise in exosome biology to deliver reliable, publication-ready data. Whether your focus is basic research, therapeutic development, or regulatory submission, our tailored solutions ensure precision and reproducibility. Contact us to discuss your project and discover how our Cryo-EM services can accelerate your research.
References
- Kwon Y, Park J. Methods to analyze extracellular vesicles at single particle level. Micro and Nano Systems Letters. 2022, 10(1): 14.
- Welsh J A, Goberdhan D C I, O'Driscoll L, et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. Journal of Extracellular Vesicles. 2024, 13(2): e12404.
- Parra A, Barranco I, Martínez-Díaz P, et al. Cryogenic electron microscopy reveals morphologically distinct subtypes of extracellular vesicles among porcine ejaculate fractions. Scientific Reports. 2024, 14(1): 16175.
Frequently Asked Questions
For any inquiries, our support team is ready to help you get technical support for your research and maximize your experience with Creative Biostructure.