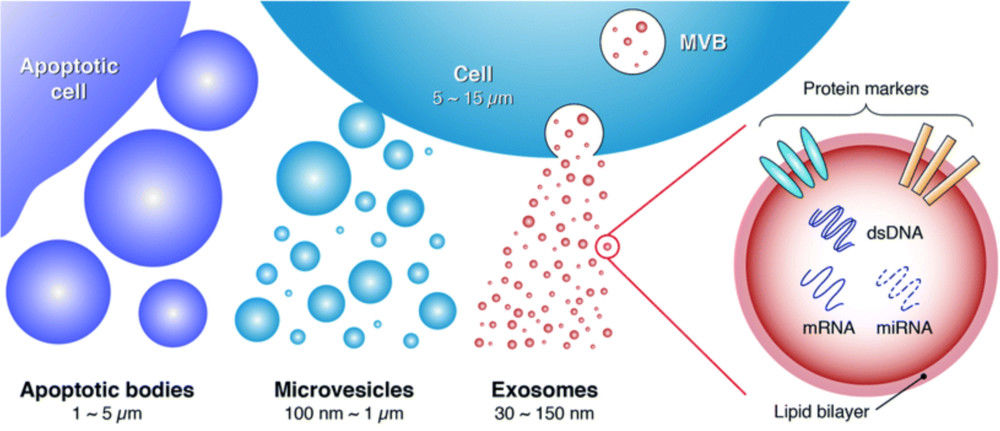
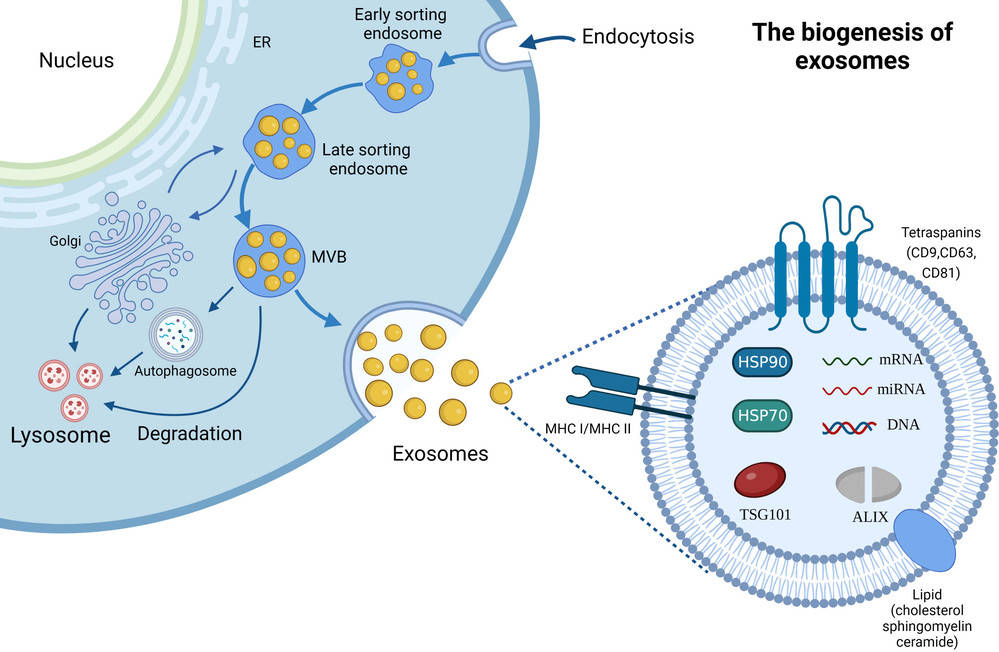
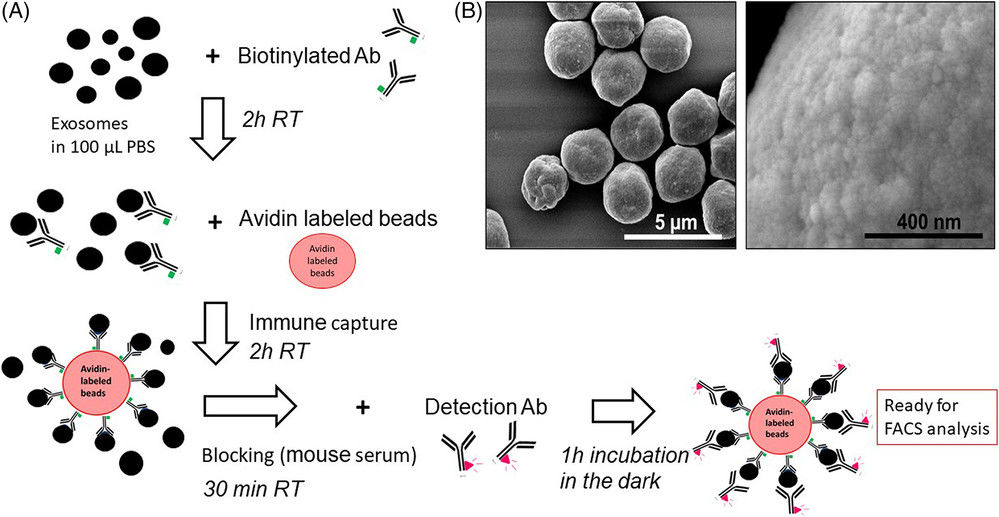
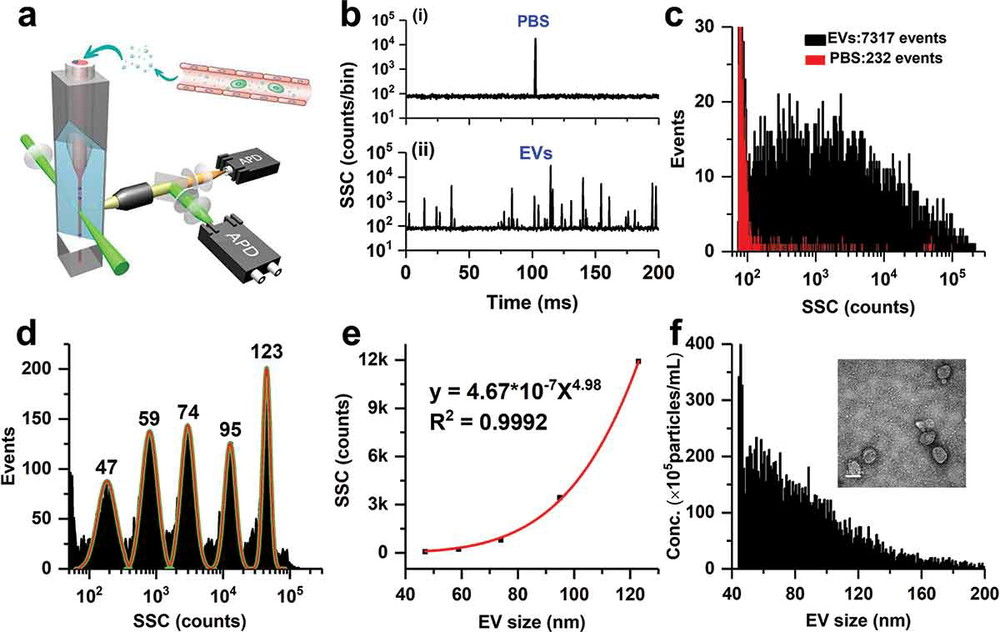
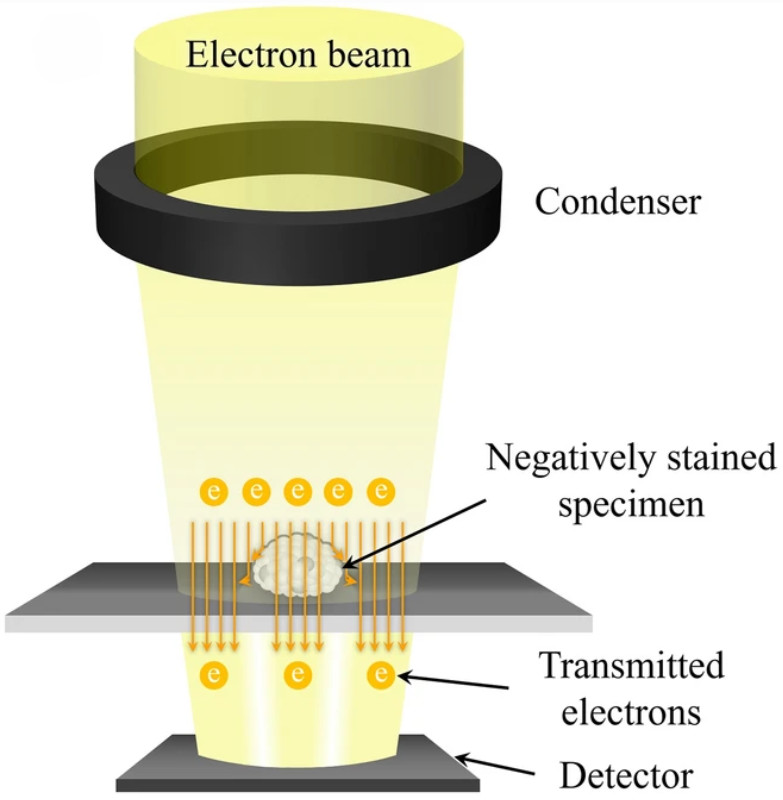
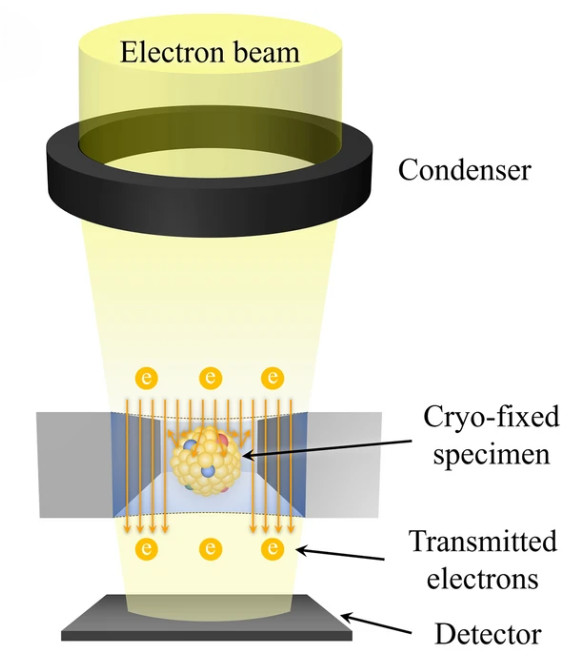
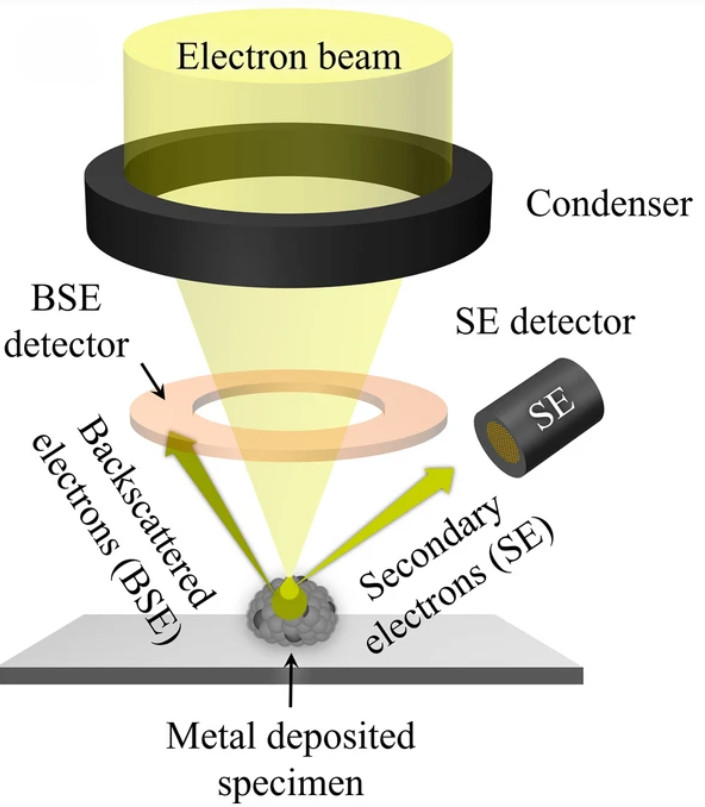
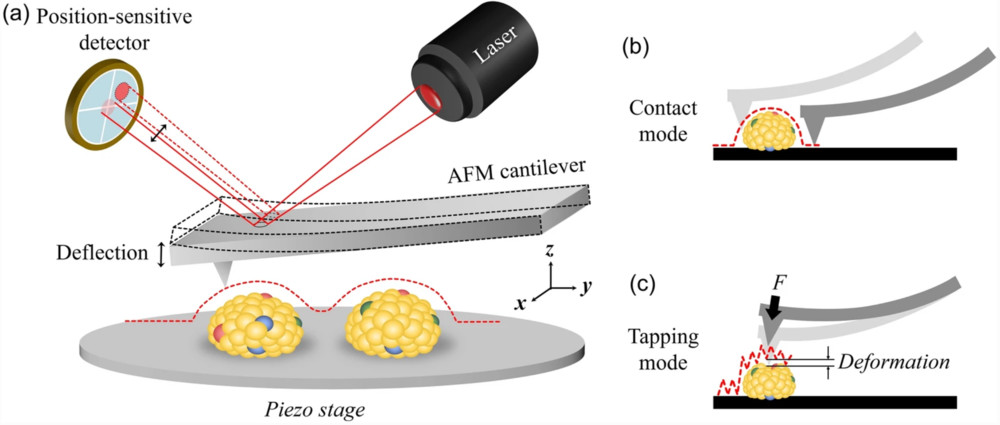
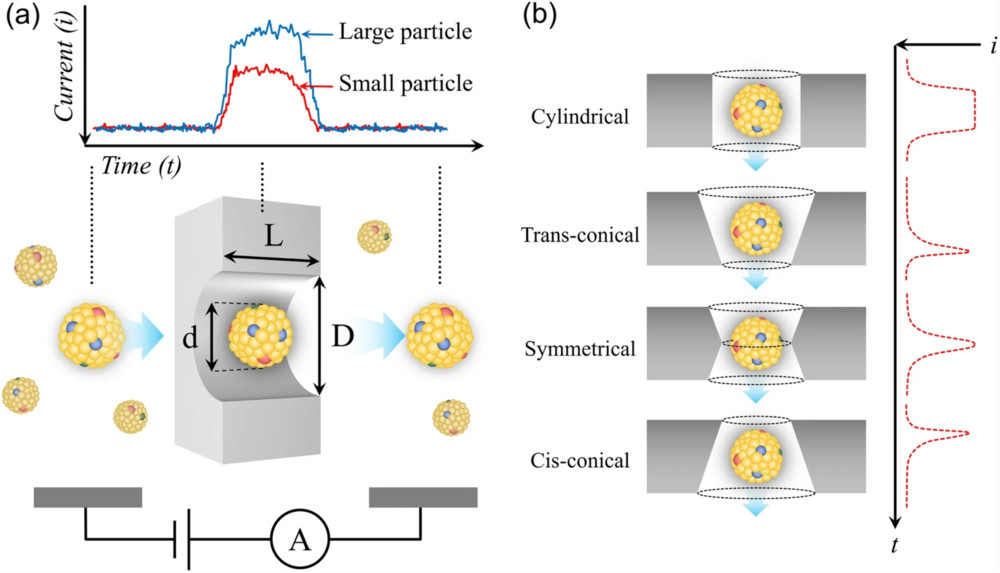
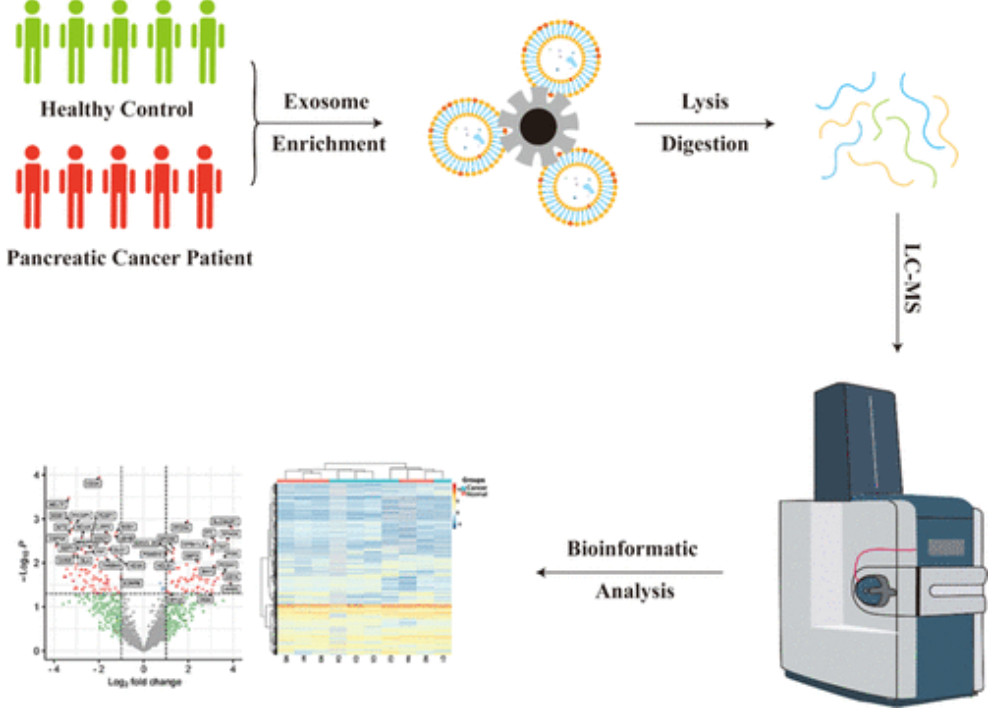
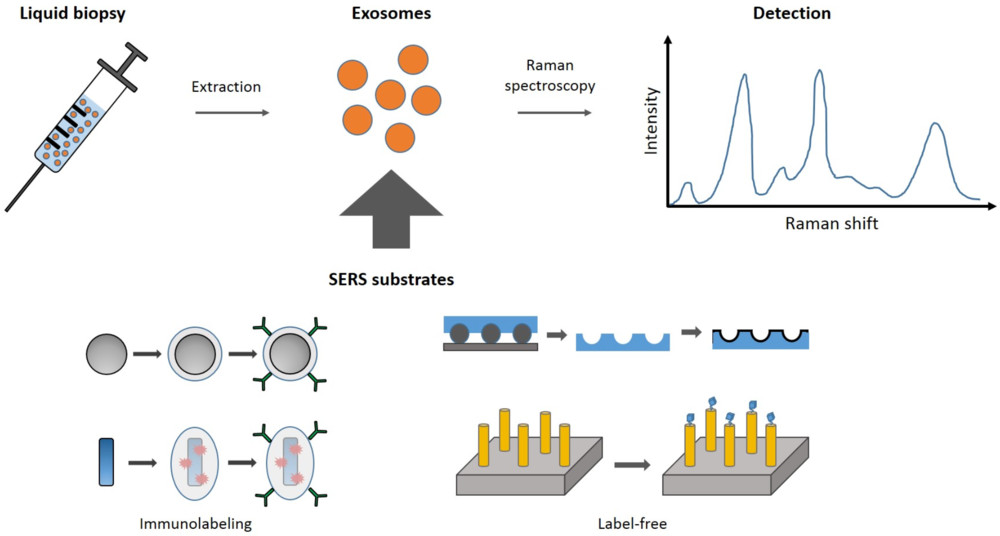
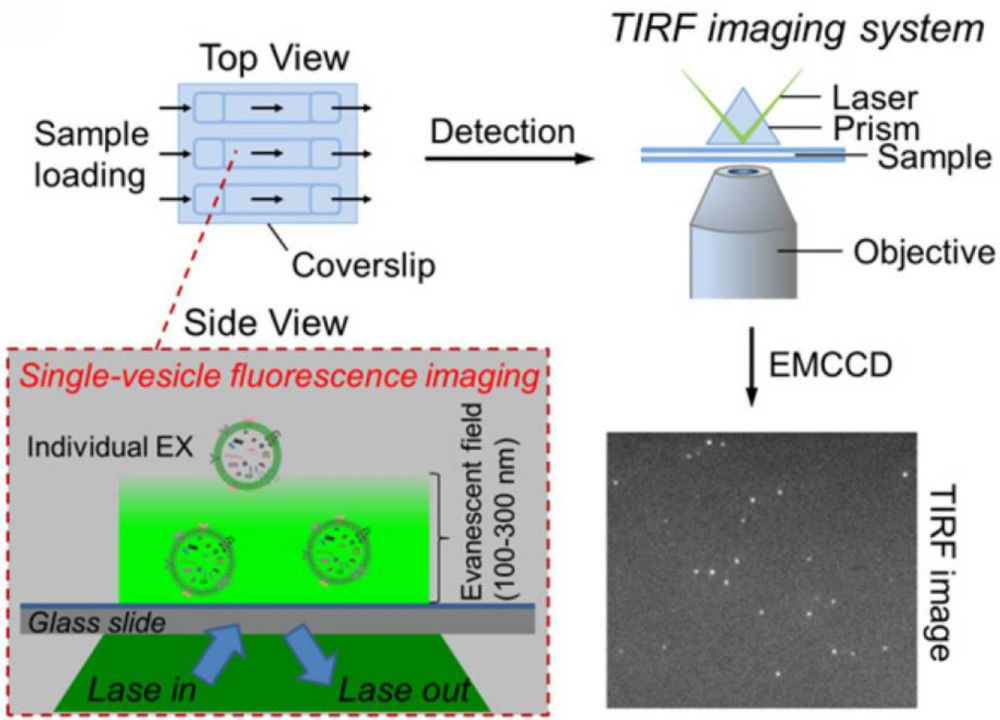
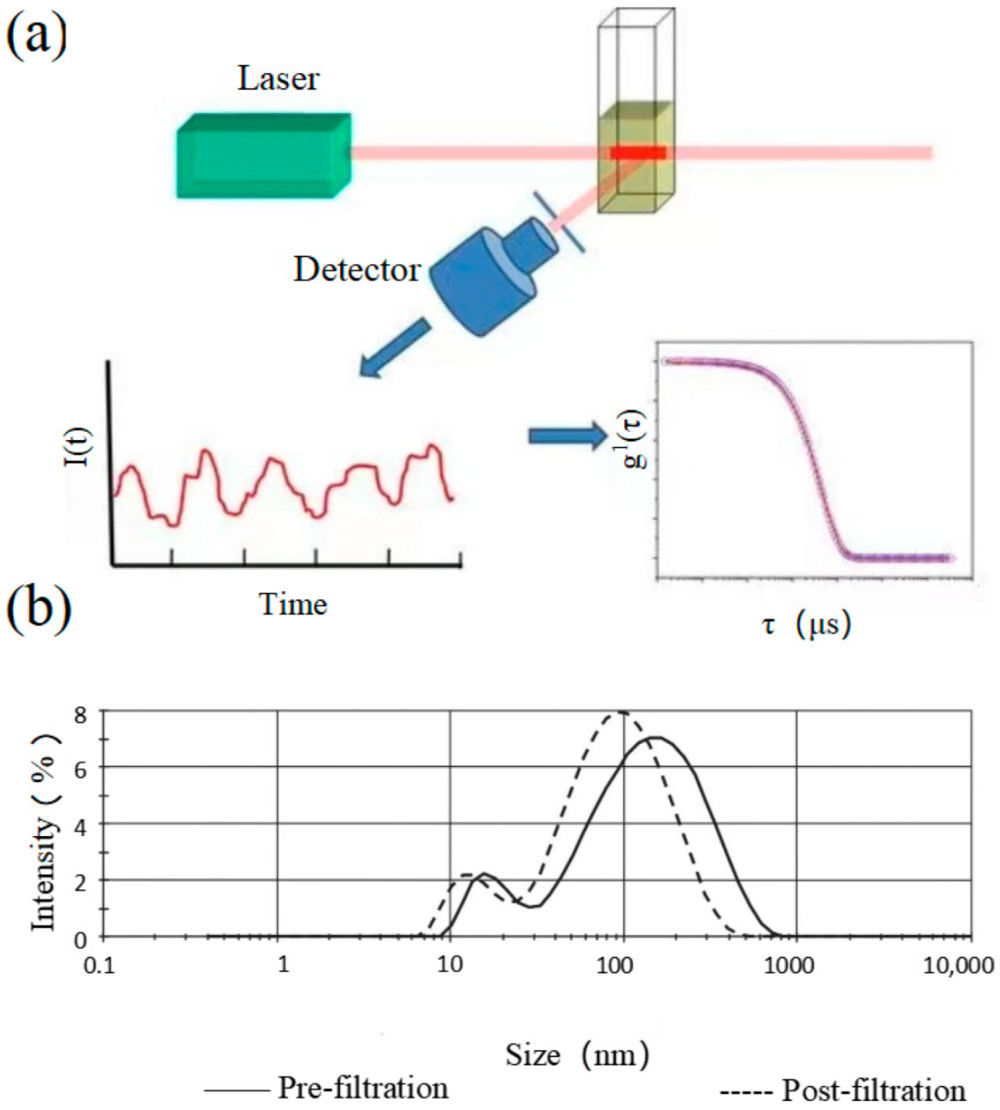
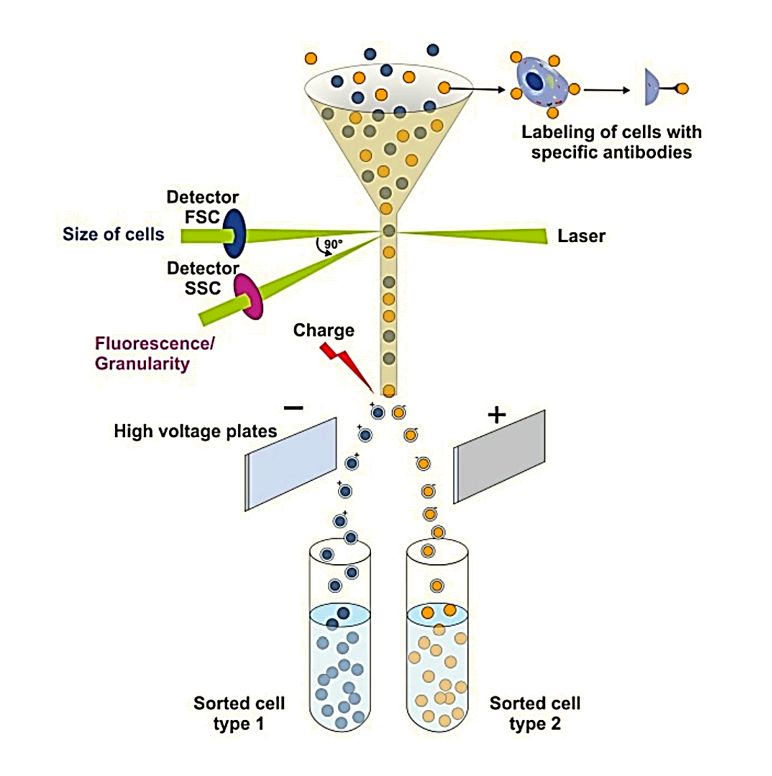
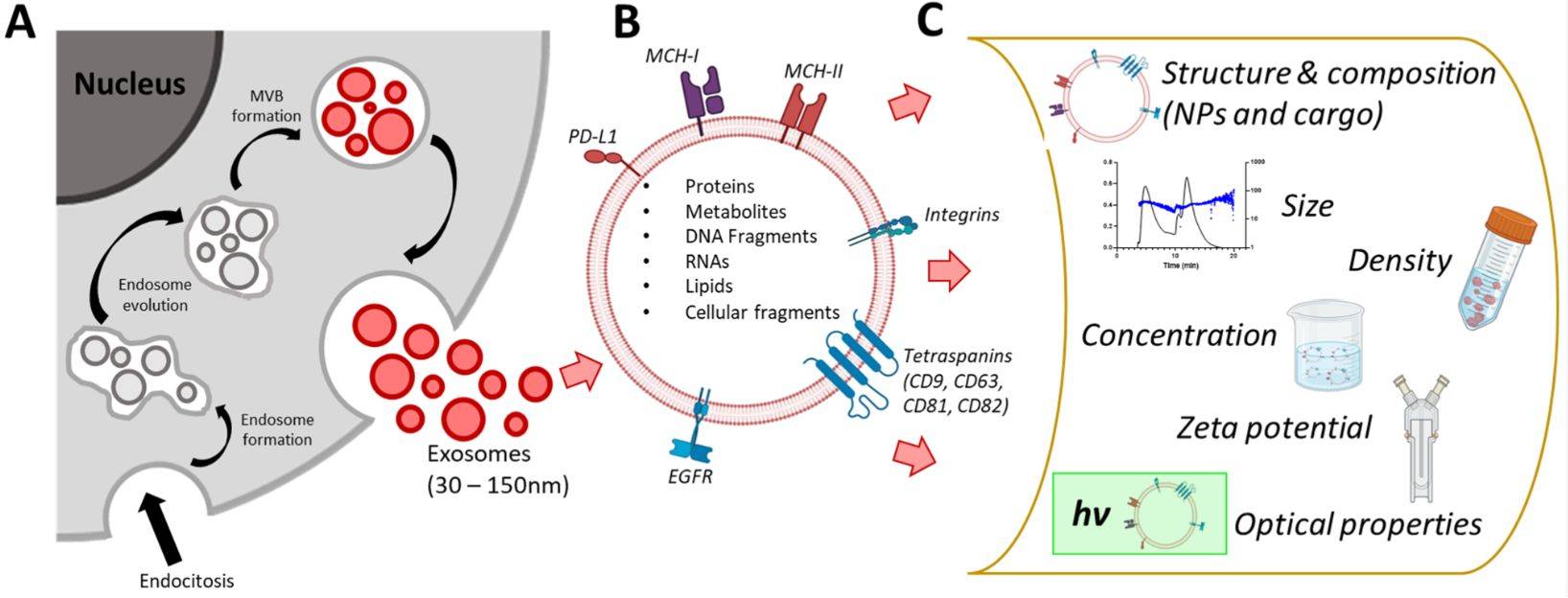
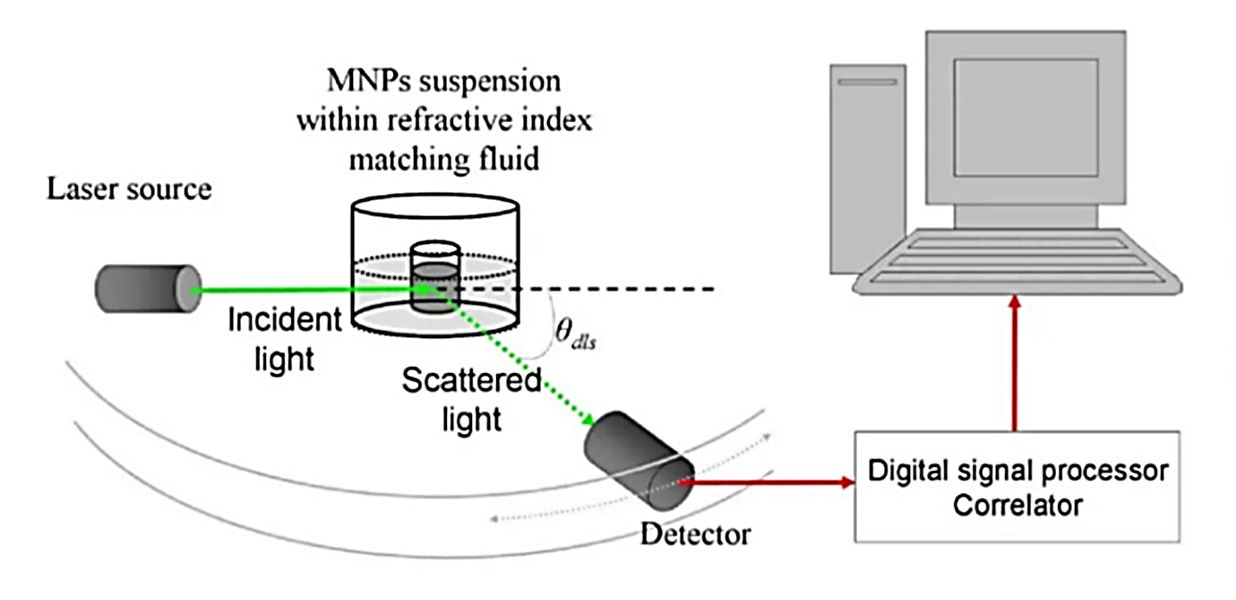
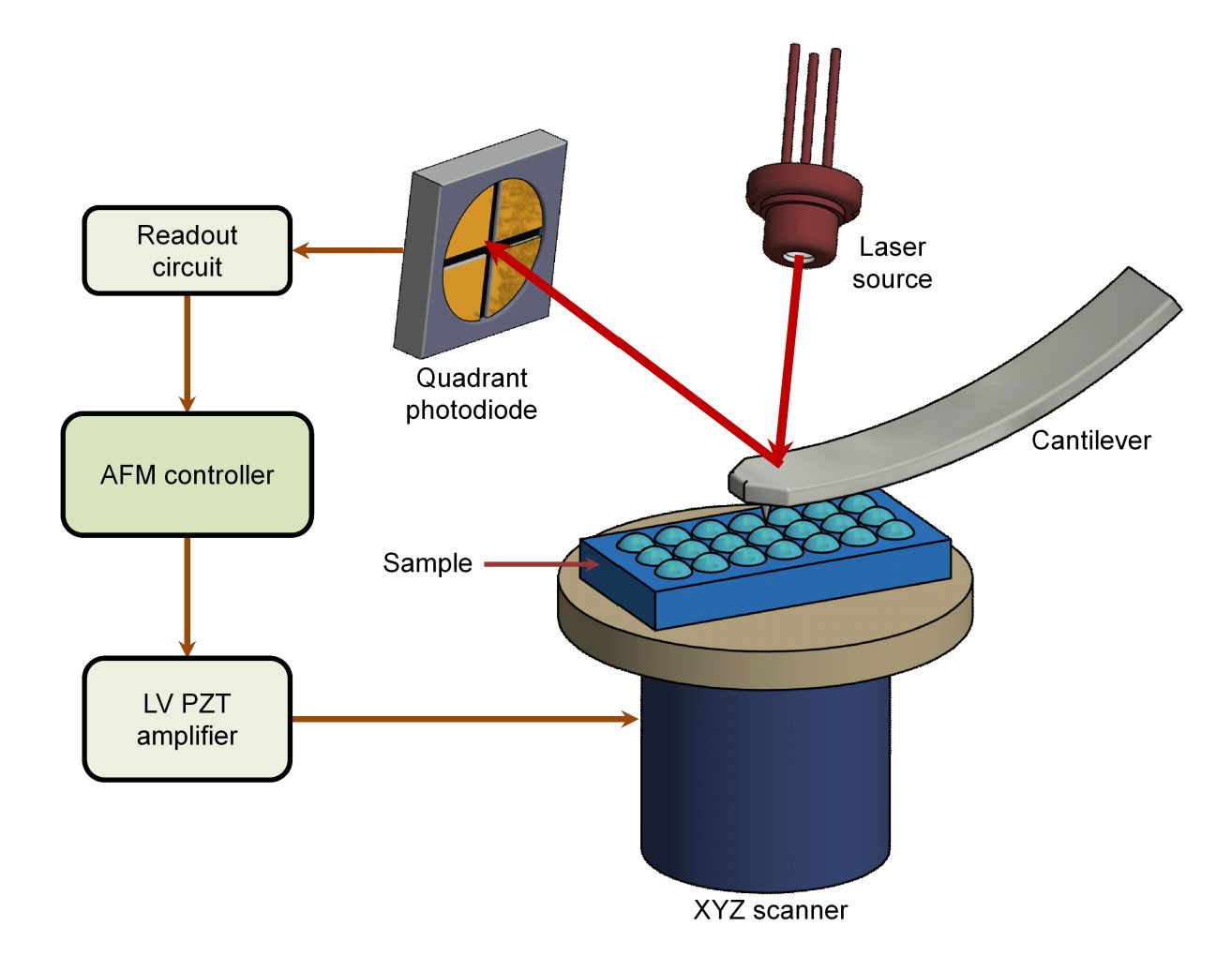
Western Blotting-Based Exosome Characterization Service
Western blotting is a gold-standard technique for validating exosome protein composition and confirming vesicle purity. By separating exosomal proteins through electrophoresis and probing with specific antibodies, researchers can identify characteristic exosome markers such as CD9, CD63, CD81, and TSG101. At Creative Biostructure, we provide a specialized Western Blotting Based Exosome Characterization Service designed to ensure accurate detection of extracellular vesicle (EV) proteins, in strict alignment with the Minimal Information for Studies of Extracellular Vesicles (MISEV2023) guidelines.
Why Use Western Blotting for Exosome Analysis?
Western blotting (immunoblotting) remains one of the most widely used methods for extracellular vesicle isolation and analysis. It enables semi-quantitative assessment of protein enrichment and provides molecular evidence for vesicle identity.
- Common exosome markers: CD9, CD63, CD81, Alix, TSG101.
- Negative controls: Calnexin and other endoplasmic reticulum proteins to exclude contamination.
- Applications: Evaluate exosome purity, confirm functional modifications, and track disease-related protein expression.
Despite its advantages, exosome Western blotting requires careful antibody selection, optimized protein loading, and inclusion of positive and negative controls. Creative Biostructure's platform addresses these challenges by combining validated protocols, high-quality antibodies, and advanced imaging systems.
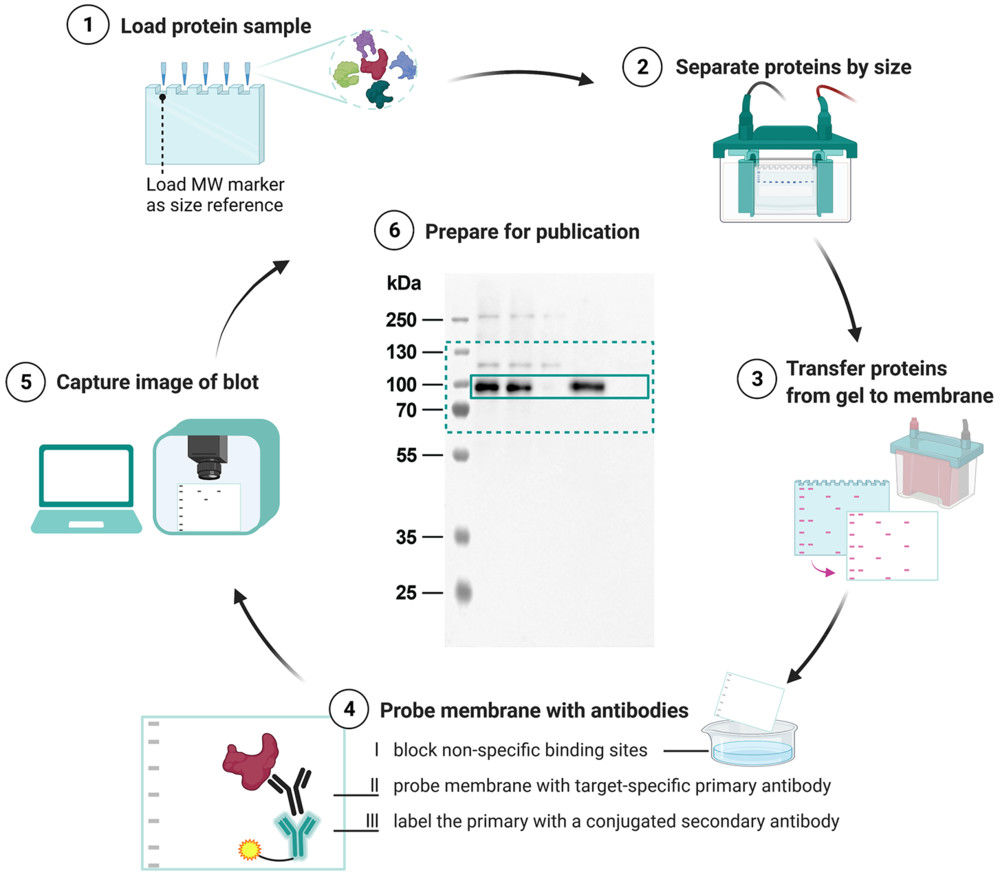 Figure 1. Schematic overview of the Western blot workflow from protein separation to final data presentation. (Kroon C, et al., 2022)
Figure 1. Schematic overview of the Western blot workflow from protein separation to final data presentation. (Kroon C, et al., 2022)
Our Exosome Western Blotting Workflow
Our exosome Western blotting protocol is carefully standardized to ensure reproducibility and compliance with international guidelines. The streamlined workflow includes the following key steps:
Exosome Isolation & Protein Preparation
Exosomes are purified from cell culture media or biofluids using ultracentrifugation, size-exclusion chromatography, or immunoaffinity capture. Proteins are then extracted and quantified (e.g., by BCA assay) to ensure consistent input.
SDS-PAGE Gel Electrophoresis
Exosomal proteins are separated based on molecular weight, generating distinct bands for subsequent identification.
Membrane Transfer
Proteins are transferred onto PVDF or nitrocellulose membranes under optimized reducing and denaturing conditions.
Antibody Probing
The membranes are incubated with validated antibodies against common exosome markers (e.g., CD9, CD63, CD81, TSG101) along with negative controls to confirm vesicle purity.
Detection & Imaging
Protein bands are visualized using advanced chemiluminescence imaging systems, providing high sensitivity for low-abundance proteins.
Data Analysis & Reporting
Band intensity is analyzed for semi-quantitative comparison. A comprehensive report is provided, including uncropped blot images, normalization details, and reproducibility assessment.
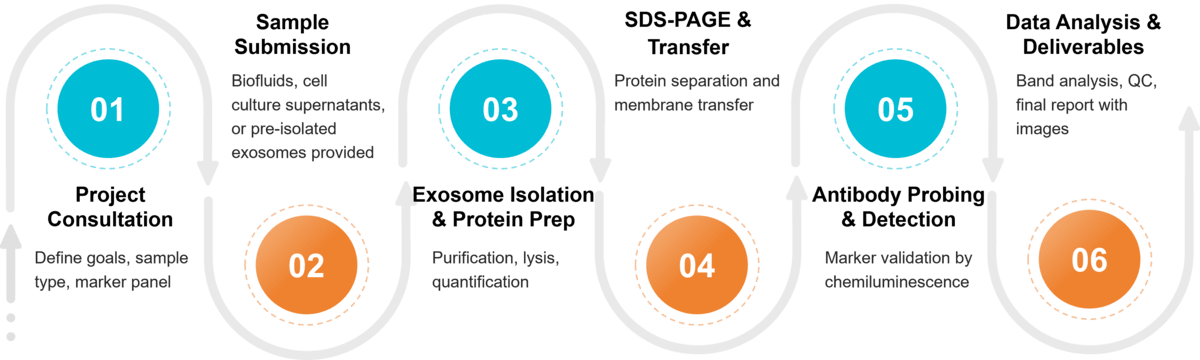 Figure 2. Project Workflow for Exosome Characterization by Western Blotting. (Creative Biostructure)
Figure 2. Project Workflow for Exosome Characterization by Western Blotting. (Creative Biostructure)
Sample Requirements for Western Blot Assay
| Requirement | Recommended Amount | Notes |
|---|---|---|
| Exosome Concentration | ≥ 1 × 1010 particles/mL | Minimum 40 µL per marker |
| Protein Input (BCA quantification) | ≥ 10 µg per marker | Protein concentration ≥ 0.25 µg/µL |
| Sample Types | Cell culture supernatant, plasma, serum, urine, CSF, tears | Contact us for other sources |
| Controls | Positive and negative controls encouraged | Antibody details should be provided if custom markers are required |
| Storage & Shipping | Store at -80 °C; ship on dry ice | Avoid repeated freeze-thaw cycles |
What Deliverables Will You Receive
- Detailed experimental workflow and normalization strategy.
- High-resolution Western blot images (cropped and uncropped).
- Marker identification results with quantitative band intensity analysis.
- Quality control assessment and reproducibility check.
- Optional bioinformatics/statistical analysis for comparative studies.
Applications of Exosome Western Blotting
- Exosome Purity Verification: Distinguish vesicles from potential contaminants.
- Disease Mechanism Research: Analyze exosome protein expression in cancer, neurodegeneration, cardiovascular, and immune disorders.
- Drug Delivery & Engineering Validation: Confirm drug loading or surface modifications in engineered exosomes.
- Quality Control in Therapeutics: Provide essential protein characterization for preclinical studies and product development.
Why Choose Creative Biostructure?
- Scientific Rigor & Compliance: Standardized workflows aligned with MISEV2023 guidelines, supported by positive/negative controls and uncropped blot images to ensure reproducibility and transparency.
- Advanced Detection & Antibody Resources: High-sensitivity chemiluminescent imaging combined with a broad panel of validated exosome marker antibodies across multiple species.
- Customized & Flexible Solutions: Tailored marker panels and experimental workflows designed to meet specific project needs in both academic and industrial research.
- Integrated Characterization Platform: Western blotting complemented by NTA, TEM, FACS, and TRPS, providing a comprehensive and trusted solution for exosome analysis.
Case Study
Case: Exosome-Mediated Delivery of IL-10 mRNA in Atherosclerosis
Background
Researchers engineered a miR-155-responsive IL-10 mRNA and encapsulated it into exosomes for targeted atherosclerosis therapy.
Methods & Results
Western blotting confirmed exosome identity with positive markers (TSG101, CD9) and absence of contaminants (GM130, APOA1). In M1 macrophages, Western blot detected robust IL-10 protein induction after exosome uptake. In ApoE-/- mice, treatment with engineered exosomes elevated IL-10 in plaques, reduced pro-inflammatory cytokines, and significantly decreased lesion size, with no systemic toxicity.
Results
TRPS showed USC-Exos were mainly 50-100 nm with a concentration of 5.1 × 1010 particles/mL, consistent with TEM findings. Marker profiling confirmed purity. Functionally, miR-21-5p-rich exosomes enhanced neuronal differentiation and improved motor and cognitive function in RTT mice.
Conclusion
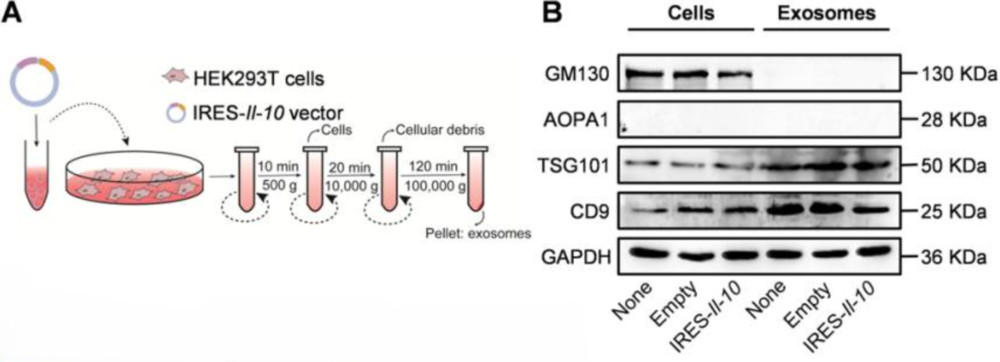 Figure 3. Preparation and Characterization of ExoIRES-IL-10. (A) Schematic illustration of the preparation and isolation process of ExoIRES-IL-10.
Figure 3. Preparation and Characterization of ExoIRES-IL-10. (A) Schematic illustration of the preparation and isolation process of ExoIRES-IL-10.
(B) Western blot analysis confirming exosome purity and marker expression. Positive exosomal markers (TSG101, CD9) were detected, while negative markers (APOA1) and exclusive marker (GM130) validated the absence of contaminants. (Bu T, et al., 2021)
Western blotting was critical for verifying vesicle purity and therapeutic protein expression, ensuring the reliability of engineered exosomes as targeted anti-inflammatory carriers.
At Creative Biostructure, we deliver rigorous and customized Western blotting-based exosome characterization services that meet the highest scientific standards. Whether you aim to validate exosome purity, confirm biomarker expression, or support therapeutic development, our expert team is ready to assist. Contact us to discuss your project and receive a tailored solution.
References
- Bu T, Li Z, Hou Y, et al. Exosome-mediated delivery of inflammation-responsive Il-10 mRNA for controlled atherosclerosis treatment. Theranostics. 2021, 11(20): 9988.
- Kroon C, Breuer L, Jones L, et al. Blind spots on western blots: Assessment of common problems in western blot figures and methods reporting with recommendations to improve them. PLoS Biology. 2022, 20(9): e3001783.
- Welsh J A, Goberdhan D C I, O'Driscoll L, et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. Journal of Extracellular Vesicles. 2024, 13(2): e12404.
Frequently Asked Questions
For any inquiries, our support team is ready to help you get technical support for your research and maximize your experience with Creative Biostructure.