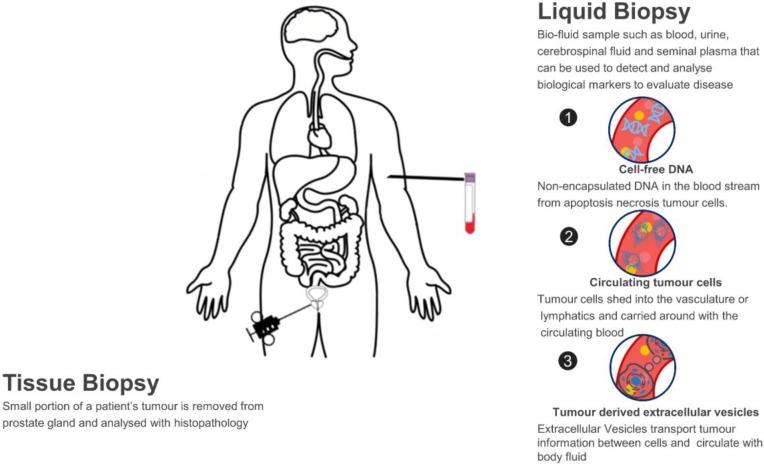
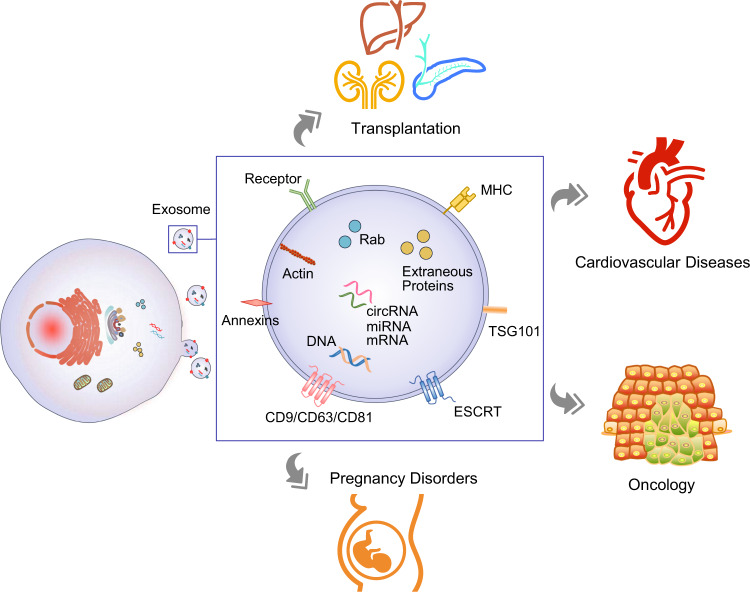
Custom Exosome Panels for Disease Biomarkers
A single biomarker is rarely enough to capture the complexity of cancer or metabolic disease. To achieve the high sensitivity and specificity required for clinical diagnostics, you need a multi-analyte approach. However, translating a list of NGS hits into a reliable, multiplexed assay is a significant engineering challenge.
We provide specialized Custom Exosome Panel Development services. We take your candidate biomarkers—whether miRNA, protein, or a combination of both—and validate them into a robust, multiplexed diagnostic panel. From assay design to clinical cohort validation, we help you build a precision tool that outperforms single-marker tests.
Why Build a Multi-Analyte Exosome Panel?
Diagnostic accuracy relies on overcoming patient heterogeneity. Custom panels offer a "fingerprint" approach that minimizes false negatives.
- Superior Diagnostic Power (AUC): Combining 3-5 biomarkers into a single panel often raises the Area Under the Curve (AUC) from mediocre (0.7) to clinical-grade (>0.9), significantly reducing misdiagnosis.
- Addressing Heterogeneity: Tumors are diverse. One patient might overexpress Marker A, while another overexpresses Marker B. A panel captures both, ensuring broader coverage of the patient population.
- Multi-Omics Synergy: We can design hybrid panels that detect both RNA (for dynamic regulation) and Protein (for functional state) simultaneously, providing a more comprehensive view of the disease pathology.
- Cost-Effective Scalability: Once validated, a targeted panel (e.g., via targeted NGS or dPCR) is far faster and cheaper to run in a clinical setting than whole-transcriptome sequencing.
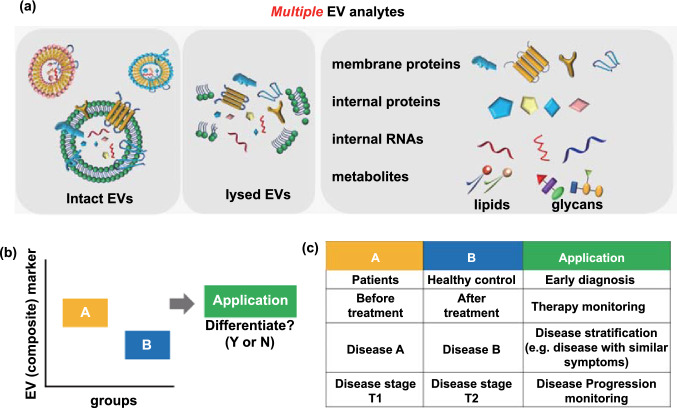 Figure 1. Composite EV biomarkers in clinical applications. (a) EV profiling by proteomics, RNAs, and metabolites. (b) Box plot of EV component expression in groups A and B. (c) Clinical uses of EV markers. (Jiang C, et al., 2021)
Figure 1. Composite EV biomarkers in clinical applications. (a) EV profiling by proteomics, RNAs, and metabolites. (b) Box plot of EV component expression in groups A and B. (c) Clinical uses of EV markers. (Jiang C, et al., 2021)
Our Panel Development Workflow
We offer a systematic pipeline to transform raw discovery data into a validated prototype assay.
| Development Phase | Our Specialized Approach & Solution | Key Services Applied |
|---|---|---|
| Candidate Selection & Filtering | In Silico Optimization: We don't just test everything. Our bioinformaticians use machine learning algorithms to select the smallest subset of markers that provides the maximum predictive power, reducing the complexity of the final assay. | Exosome Multi-Omics Integration, Extracellular Vesicle Biomarker Discovery |
| Assay Design & Multiplexing | Method Development: We optimize the chemistry for multiplexing. Whether it's designing non-interfering primers for a Multiplex dPCR assay or selecting compatible antibody pairs for a Luminex/Simoa protein array, we ensure zero cross-reactivity. | Exosome Surface Marker Analysis |
| Analytical Validation | Robustness Testing: We rigorously test the panel's performance limits. We determine the Limit of Detection (LOD), linearity, and reproducibility across different days and operators to ensure the assay is rugged enough for clinical use. | Exosome Purity Analysis, Exosome Quality Control |
| Clinical Cohort Validation | Blind Testing: We validate the panel in an independent cohort of patient samples (case vs. control). We calculate the final Sensitivity, Specificity, and Positive Predictive Value (PPV) to demonstrate clinical utility. | Liquid Biopsy via Exosomal miRNA/Protein |
Core Technologies for Panel Validation
We utilize platforms that allow for precise, simultaneous quantification of multiple targets.
Digital PCR (dPCR) for Absolute Quantification
Precision RNA Panels: For miRNA or gene expression signatures, digital PCR is the gold standard. It partitions the sample into thousands of droplets, allowing for the absolute quantification of multiple targets with higher resistance to inhibitors than standard qPCR. This is ideal for validating low-abundance exosomal RNA panels.
Multiplex Immunoassays
High-Throughput Protein Panels: When your panel involves multiple surface proteins, we use bead-based multiplex assays or electrochemiluminescence. These technologies allow us to quantify up to 10-plex or more proteins in a single well, conserving precious patient samples while delivering broad proteomic data.
Targeted NGS Panels
High-Content Screening: For panels requiring tens or hundreds of markers (e.g., a comprehensive oncology panel), we design custom Targeted NGS Library Prep kits. This focuses sequencing depth only on your genes of interest, dramatically lowering costs while maintaining high sensitivity.
Application Spotlight: A Multi-miRNA Panel for Early Lung Cancer Detection
This analysis demonstrates how combining multiple exosomal markers into a single panel significantly boosts diagnostic accuracy compared to single biomarkers.
Featured Technologies:
- Plasma Exosome Isolation
- Custom Panel Design & Validation
Literature Interpretation:
Early detection of lung adenocarcinoma (LUAD) is critical, but traditional tumor markers lack sensitivity. Single miRNA markers often fail due to patient heterogeneity. Researchers utilized a discovery-to-validation workflow to identify a specific panel of markers. They screened plasma exosomes and identified a specific 3-miRNA signature (miR-103b, miR-877-5p, and miR-29c-5p).
While individual miRNAs showed moderate diagnostic ability, the combined miRNA panel achieved a remarkable Area Under the Curve (AUC) of 0.985 in the validation cohort, significantly outperforming any single marker. This outcome validates the core philosophy of constructing multi-analyte signatures is the most effective strategy to achieve the high sensitivity and specificity required for clinical-grade diagnostics.
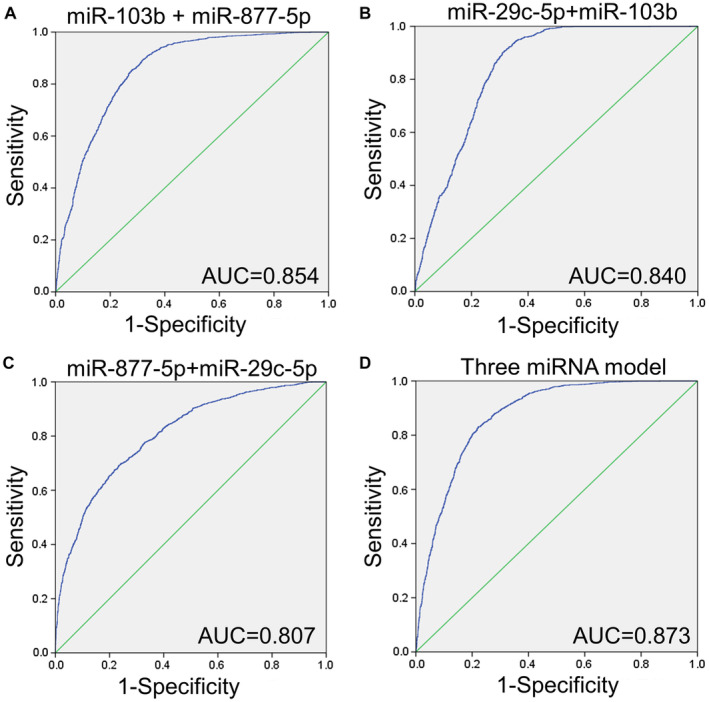 Figure 2. Validation of miRNA panels as biomarkers for lung cancer diagnosis. Panels (A) through (D) represent different combinations of miRNAs: hsa-miR-877-5p + hsa-miR-103b, hsa-miR-29c-5p + hsa-miR-103b, hsa-miR-877-5p + hsa-miR-29c-5p, and the combination of all three miRNAs. The ROC curve from the microarray GSE137140 is depicted in blue. (Wu J, et al., 2022)
Figure 2. Validation of miRNA panels as biomarkers for lung cancer diagnosis. Panels (A) through (D) represent different combinations of miRNAs: hsa-miR-877-5p + hsa-miR-103b, hsa-miR-29c-5p + hsa-miR-103b, hsa-miR-877-5p + hsa-miR-29c-5p, and the combination of all three miRNAs. The ROC curve from the microarray GSE137140 is depicted in blue. (Wu J, et al., 2022)
Start Your Panel Development Project
We make getting started straightforward. Our process is designed to be collaborative and transparent.
How It Works: Our Project Pathway
 Figure 3. Our systematic workflow for converting candidate biomarkers into a robust, multiplexed diagnostic panel. (Creative Biostructure)
Figure 3. Our systematic workflow for converting candidate biomarkers into a robust, multiplexed diagnostic panel. (Creative Biostructure)
Ready to turn your biomarker list into a validated product? Our scientific team is available for a free consultation to discuss your diagnostic panel strategy. Contact us today to discuss your project.
References
- Jiang C, Fu Y, Liu G, et al. Multiplexed Profiling of Extracellular Vesicles for Biomarker Development. Nanomicro Lett. 2021 Dec 2;14(1):3.
- Wu J, Feng Z, Wang R, et al. Integration of bioinformatics analysis and experimental validation identifies plasma exosomal miR-103b/877-5p/29c-5p as diagnostic biomarkers for early lung adenocarcinoma. Cancer Med. 2022 Dec;11(23):4411-4421.