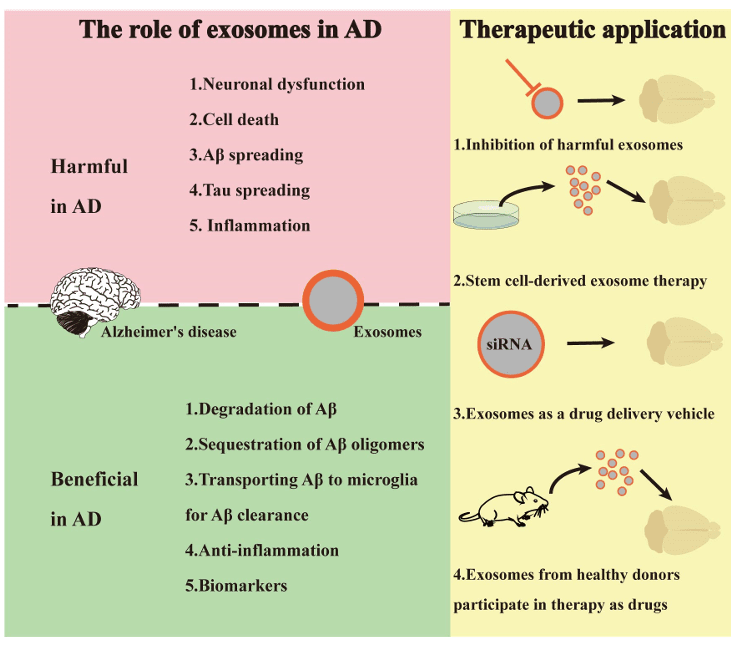
Parkinson Disease Exosome Research Services
Parkinson's Disease (PD) is characterized by the accumulation and prion-like spread of misfolded alpha-Synuclein, leading to the selective loss of dopaminergic neurons. Early diagnosis and monitoring of disease progression remain major unmet needs. Neuron-derived exosomes (NDEs) provide an invaluable window into the brain, carrying pathological markers like phosphorylated alpha-Synuclein (pS129) from the substantia nigra to peripheral biofluids long before motor symptoms appear.
We provide specialized Parkinson Disease Exosome Research solutions. We focus on the enrichment of neural exosomes using L1CAM/CD47 capture technology and the precision measurement of pathological targets via Quantitative Proteomics. Whether you are developing blood-based diagnostic markers or investigating the prion-like propagation mechanisms using Protein Interaction Assays, our platform provides the sensitivity and functional depth required for neuro-discovery.
Critical Frontiers in Pre-Clinical PD Research
Parkinson's Disease research focuses on early stratification and halting the spread of alpha-Synuclein.
- Prodromal Detection: By the time motor symptoms appear, significant dopaminergic neuron loss has already occurred. Identifying biomarkers in the prodromal phase (e.g., during REM sleep behavior disorder) is critical for early intervention.
- Distinguishing PD Subtypes: Clinically differentiating PD from atypical parkinsonism (like MSA or PSP) is difficult. Molecular profiling of neuron-derived exosomes seeks to provide objective differential diagnosis.
- Alpha-Synuclein Spreading: The "Prion Hypothesis" suggests pathological alpha-Synuclein spreads from cell to cell. Validating the role of exosomes as the carriers of these toxic seeds is key to developing disease-modifying therapies.
- Lysosomal Dysfunction: Genetic links to GBA1 and LRRK2 implicate lysosomal failure. Research investigates how exosomes serve as an alternative pathway for clearing protein aggregates when lysosomes fail.
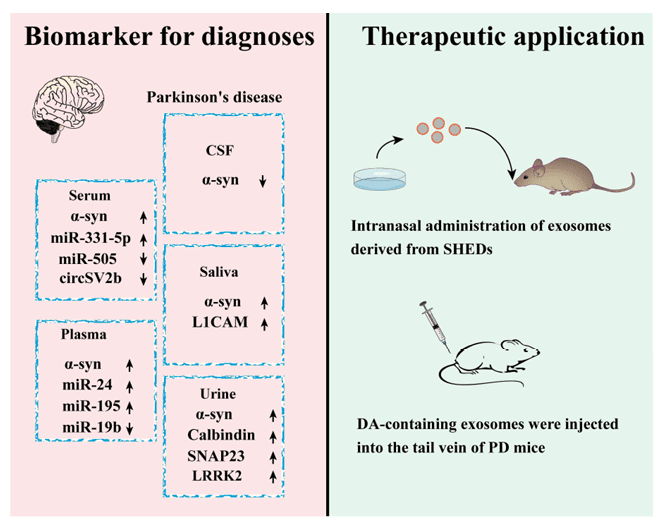 Figure 1. Exosome-based diagnostics and therapeutic strategies for Parkinson's disease (PD), highlighting biomarker detection and treatment delivery methods. (He A, et al., 2023)
Figure 1. Exosome-based diagnostics and therapeutic strategies for Parkinson's disease (PD), highlighting biomarker detection and treatment delivery methods. (He A, et al., 2023)
Our PD Research Workflow
We offer a pipeline optimized for low-abundance neural markers and functional propagation studies.
| Research Phase | Our Specialized Approach & Solution | Key Services Applied |
|---|---|---|
| Neural Enrichment | L1CAM / CD47 Capture: Since neural exosomes are rare in blood, we use Anti-L1CAM or Anti-CD47 Immunomagnetic Beads to selectively capture neuron-derived vesicles, maximizing the signal-to-noise ratio for brain-specific markers. | Exosome Isolation by Immunoaffinity Capture |
| Pathological Quantification | Targeted Protein Quant: We utilize Exosome Quantitative Proteomicsto perform absolute quantification of specific pathological markers (like pS129-alpha-Syn) at femtogram levels. For broader discovery of co-regulated synaptic proteins, we apply Biomarker Protein Screening. | Exosome Quantitative Proteomics, Exosome Biomarker Protein Screening |
| Propagation Modeling | Seeding & Interaction: To validate the "prion-like" spread, we use Exosome-protein Interactions Assays. We track how exosomal alpha-Syn physically interacts with and induces the aggregation of endogenous proteins in recipient cells (FRET/Seeding assay). | Exosome-protein Interactions Assay |
| Neurotoxicity & Rescue | Cellular Viability: We evaluate the impact of exosomes on dopaminergic neuron health using Exosome Cellular Functional Assays. We measure critical endpoints including cell proliferation (CCK-8), apoptosis (Caspase-3), and LDH release to quantify neurotoxicity. | Exosome Cellular Functional Assays |
Core Technologies for Parkinson's Disease
We deploy specific technologies designed to handle the complexity of neurodegenerative samples.
L1CAM/CD47 Neural Exosome Isolation
Enhancing Specificity: L1CAM (CD171) is a cell adhesion molecule highly expressed on neurons. By performing Immuno-Affinity Capture targeting L1CAM, we enrich the fraction of exosomes originating from the CNS. This is critical for distinguishing brain-derived alpha-Synuclein from peripheral sources, ensuring accurate diagnostic readings.
Targeted Alpha-Synuclein Quantification
Precision Diagnostics: The concentration of pathological alpha-Syn in blood is extremely low. Under our Exosome Quantitative Proteomics service, we utilize Simoa (Single Molecule Array) technology. This allows for the absolute quantification of specific targets like Phosphorylated (pS129) alpha-Synuclein at sub-femtogram levels, far exceeding the sensitivity of standard ELISA.
Alpha-Synuclein Seeding & Interaction Analysis
TTracking the Spread: The "prion-like" spread of PD relies on the interaction between exosomal "seeds" and endogenous proteins. Using our Exosome-protein Interactions Assay platform, we perform Seeding Activity Assays. We detect the physical interaction and induced aggregation of monomeric alpha-Syn substrate by patient exosomes using FRET or Thioflavin T fluorescence.
Application Spotlight: Microglial Exosomes Drive Disease Spread
This analysis highlights how immune cells in the brain can inadvertently act as vectors for pathology via exosome secretion, validating the need for functional interaction assays.
Featured Technologies:
- Exosome-protein Interactions Assay (Seeding)
- Exosome Quantitative Proteomics
Literature Interpretation:
The progression of Parkinson's disease is attributed to the prion-like spreading of alpha-Synuclein across brain regions. This study investigated the contribution of microglia, the brain's resident immune cells, to this process. Researchers demonstrated that when microglial autophagy is impaired, these cells fail to degrade internalized alpha-Synuclein aggregates. Instead, they package the undigested pathological proteins into exosomes and release them into the extracellular space. These microglial exosomes are highly capable of being taken up by neurons, where they physically interact with endogenous alpha-Synuclein and induce its aggregation (seeding). This finding establishes microglial exosomes as potent vehicles for disease transmission and underscores the critical importance of using protein interaction assays to evaluate the seeding capacity of exosomes in neurodegenerative models.
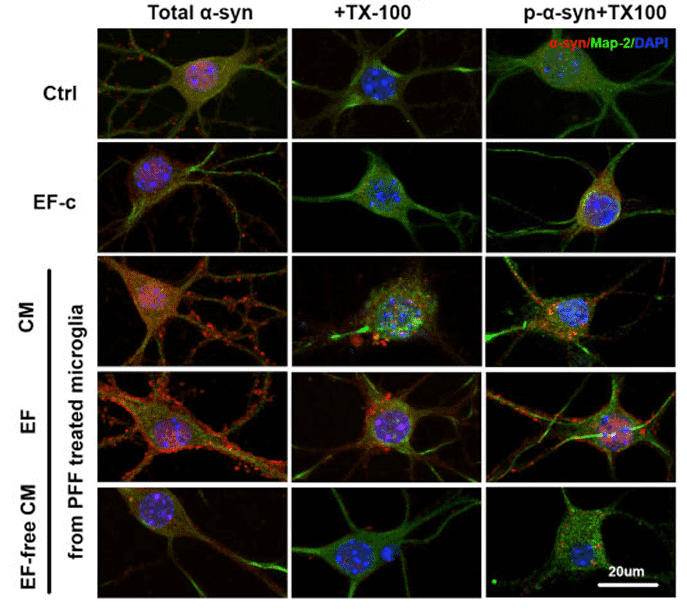 Figure 2. Effects of exosomes from PFF-treated microglia on α-synuclein aggregation in neurons, as assessed by immunostaining for total and phosphorylated α-synuclein after neuron fixation with PFA and TX-100. (Guo M, et al., 2020)
Figure 2. Effects of exosomes from PFF-treated microglia on α-synuclein aggregation in neurons, as assessed by immunostaining for total and phosphorylated α-synuclein after neuron fixation with PFA and TX-100. (Guo M, et al., 2020)
Start Your PD Research Project
Advance your research on neurodegeneration with our specialized CNS exosome platform.
How It Works: Our Project Pathway
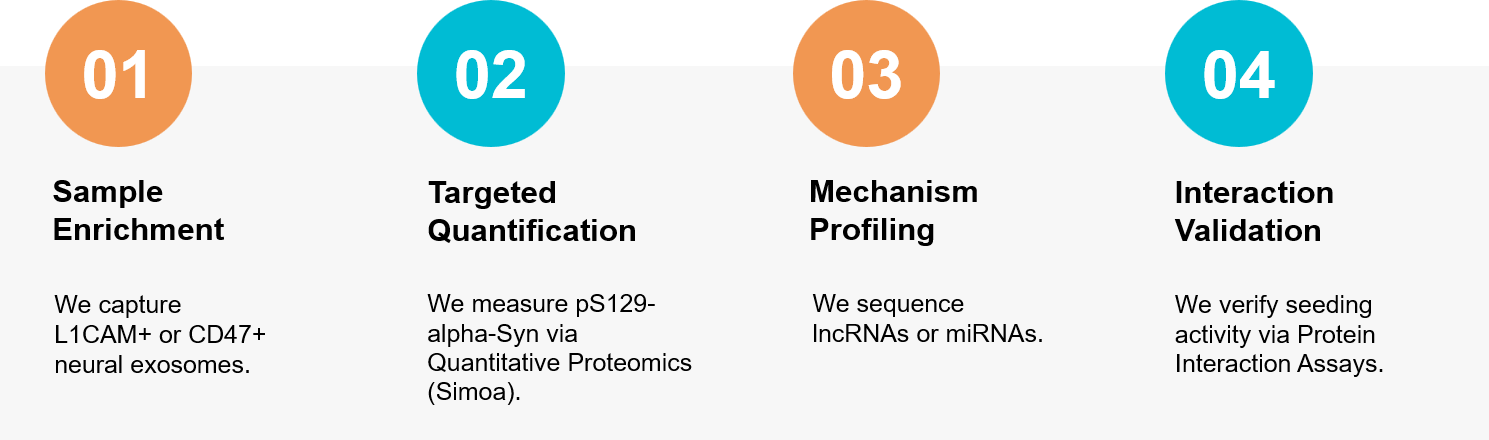 Figure 3. Workflow for isolating neural exosomes, quantifying alpha-Synuclein, and validating disease propagation. (Creative Biostructure)
Figure 3. Workflow for isolating neural exosomes, quantifying alpha-Synuclein, and validating disease propagation. (Creative Biostructure)
Ready to validate the next generation of Alzheimer's biomarkers? Our neuroscience team is available to discuss your L1CAM enrichment strategy. Contact us today to discuss your project.
References
- He A, Wang M, Li X, et al. Role of Exosomes in the Pathogenesis and Theranostic of Alzheimer's Disease and Parkinson's Disease. Int J Mol Sci. 2023 Jul 4;24(13):11054.
- Guo M, Wang J, Zhao Y, et al. Microglial exosomes facilitate α-synuclein transmission in Parkinson's disease. Brain. 2020 May 1;143(5):1476-1497.