Protozoa-Derived Extracellular Vesicle Isolation Service
At Creative Biostructure, we provide specialized protozoa-derived extracellular vesicle (EV) isolation services to support researchers studying host-pathogen interactions, vaccine development, and diagnostic biomarker discovery. Our service is designed to deliver highly pure and functionally intact EVs from various protozoan species using state-of-the-art isolation technologies and stringent quality control protocols.
Why Study Protozoa-Derived Extracellular Vesicles?
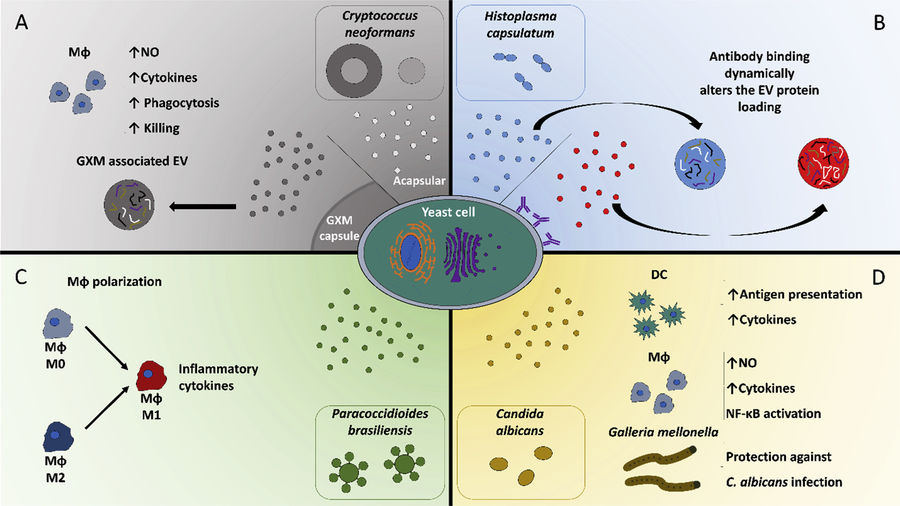
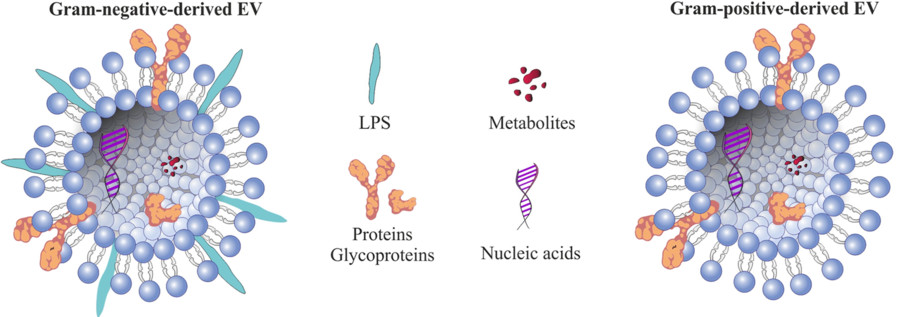
Protozoan parasites such as Leishmania, Giardia, Plasmodium, and Toxoplasma gondii actively secrete nanoscale extracellular vesicles that play essential roles in intercellular communication, immune modulation, and pathogenicity. These vesicles carry a diverse range of bioactive cargos such as proteins, RNAs, lipids, and virulence factors, all of which contribute to disease progression and host response mechanisms.
Isolating and characterizing protozoa-derived EVs is crucial for:
- Investigating mechanisms of immune evasion and infection
- Identifying novel therapeutic and vaccine targets
- Developing non-invasive diagnostic assays
- Studying cell signaling pathways in protozoan biology
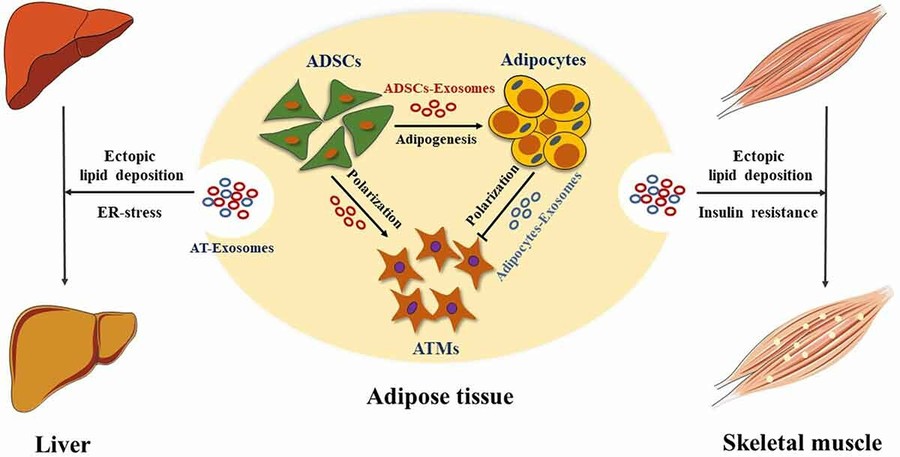
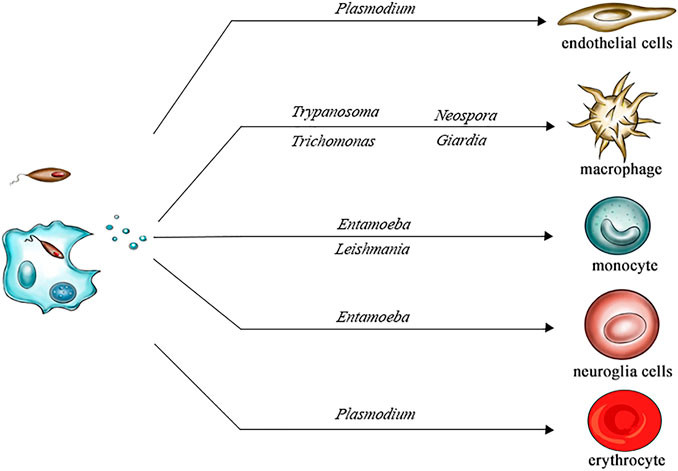 Figure 1. Cell Tropism of Parasite-Derived Extracellular Vesicles. (Wang X, et al., 2022)
Figure 1. Cell Tropism of Parasite-Derived Extracellular Vesicles. (Wang X, et al., 2022)
Our Extracellular Vesicle Isolation Technologies
To address the complexity of protozoan biology and the diverse requirements of EV research, Creative Biostructure offers a comprehensive suite of isolation techniques. Each method is selected and refined based on the biological characteristics of the source organism and the intended analytical purpose.
| Technique | Description | Applicable Applications |
|---|---|---|
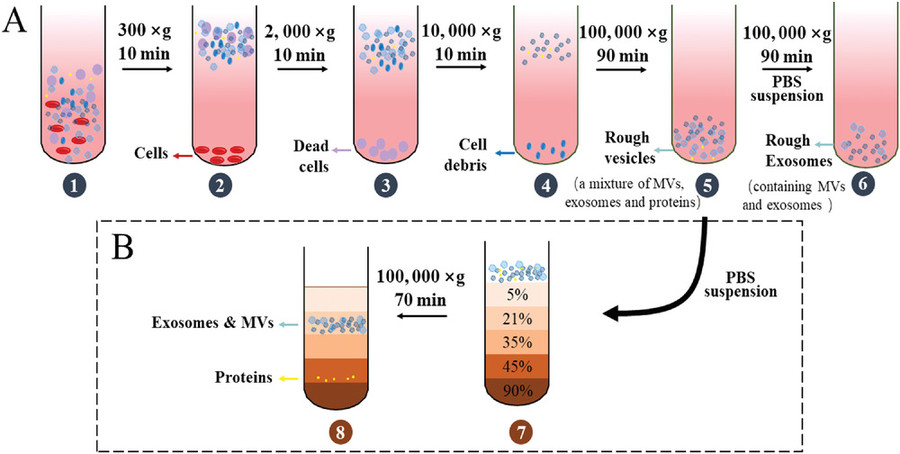
| Differential Centrifugation | Sequential centrifugation at increasing speeds to remove cells, debris, and pellet vesicles based on size and density. | General EV isolation from culture supernatants or biological fluids. |
| Ultracentrifugation | High-speed centrifugation (≥100,000 × g) to pellet small extracellular vesicles such as exosomes. | Suitable for purifying exosomes from protozoan cultures. |
| Density Gradient Centrifugation | EVs are separated by buoyant density using sucrose or iodixanol gradients to increase purity. | High-purity EV isolation for proteomics or biomarker analysis. |
| Ultrafiltration | Size-based membrane filtration to concentrate vesicles and remove small soluble molecules. | Pre-concentration of EVs for downstream purification. |
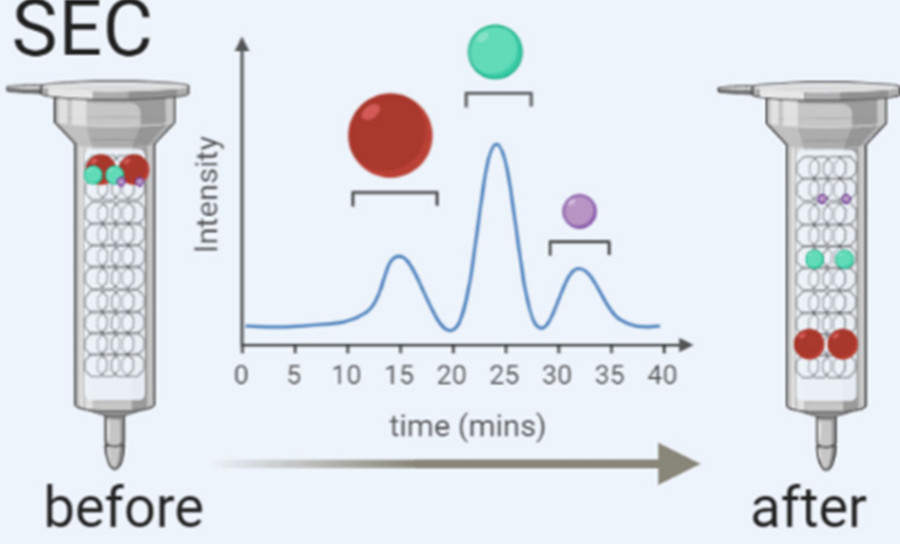
| Size-Exclusion Chromatography (SEC) | Separates EVs from protein aggregates and free biomolecules using porous matrices. | Preserves vesicle integrity for functional and structural studies. |
| Polyethylene Glycol (PEG) Precipitation | Uses PEG to aggregate and precipitate EVs from dilute solutions. | High-throughput or preliminary screening workflows. |
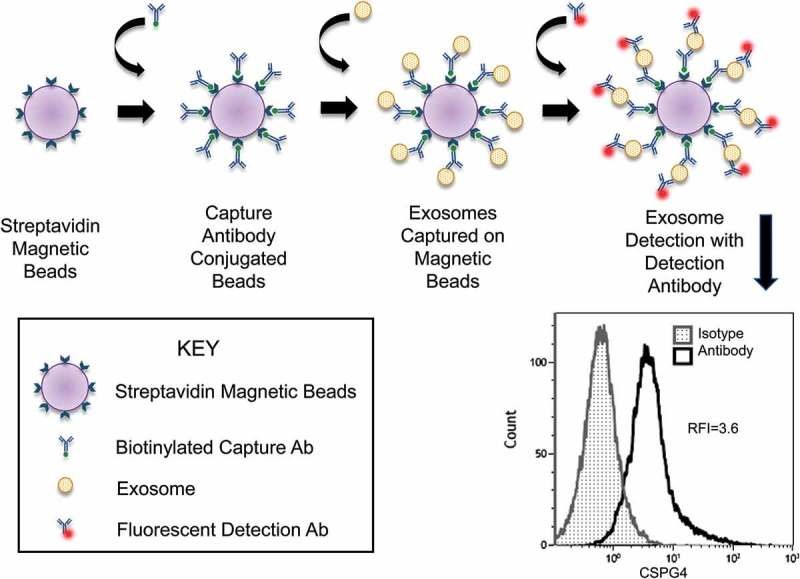
| Immunoaffinity Capture | Utilizes antibodies against specific surface markers to isolate defined EV subpopulations. | Targeted enrichment for EV subtype characterization or vaccine development. |
Our protocols can be tailored to combine multiple isolation strategies, ensuring optimal vesicle recovery and structural integrity. Whether your goal is downstream omics analysis, functional assays, or immunological profiling, we can adapt the workflow to meet your scientific needs.
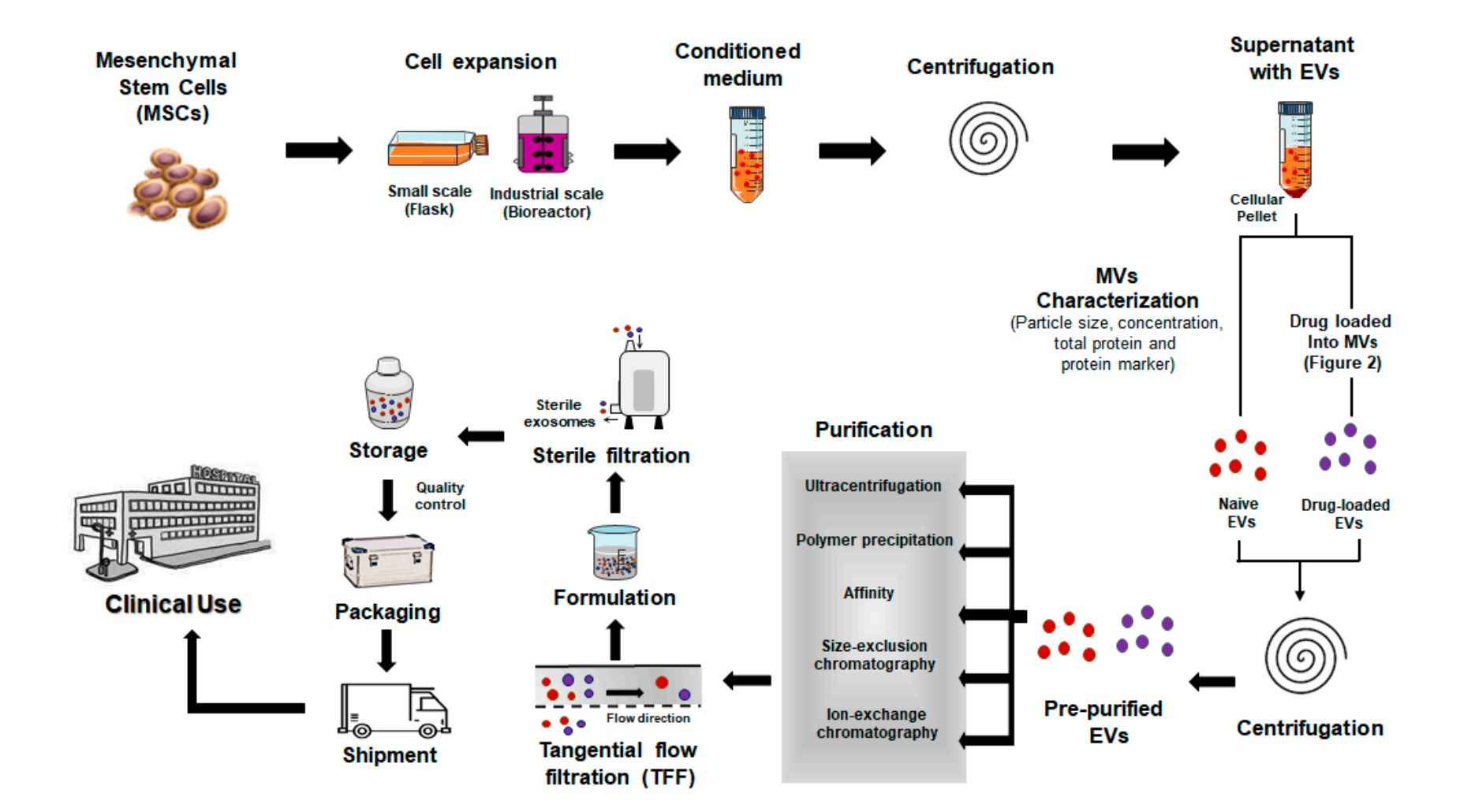
Our Protozoa-Derived Extracellular Vesicle Isolation Workflow
At Creative Biostructure, we follow a standardized yet customizable workflow to ensure the efficient and reproducible isolation of extracellular vesicles from protozoan samples:
Project Consultation
We begin with a detailed discussion to understand your research objectives, protozoan species, sample types, and downstream application needs.
Sample Submission
Customers submit either cultured protozoan supernatants or host-derived biofluids/tissue homogenates. Guidelines for sample volume and storage conditions are provided during consultation.
Pre-Processing
Samples undergo initial low-speed centrifugation and filtration to remove protozoan cells, debris, and apoptotic bodies, ensuring a clean starting material for EV isolation.
Primary EV Isolation
Depending on the sample and project requirements, we apply one or more core techniques such as differential centrifugation, ultracentrifugation, PEG precipitation, or ultrafiltration.
Optional Purification
For applications requiring higher purity, we perform secondary purification steps such as density gradient centrifugation or SEC.
Characterization & Quality Control
Isolated vesicles are evaluated using NTA, TEM, protein assays, and EV marker profiling to verify integrity, concentration, and purity.
Final Deliverables
We provide concentrated, high-quality protozoa-derived EVs in the desired buffer, along with a full technical report including workflow details, yield, and QC results.
This streamlined yet flexible workflow ensures that every project is aligned with your scientific goals and meets the quality expectations for downstream research and development.
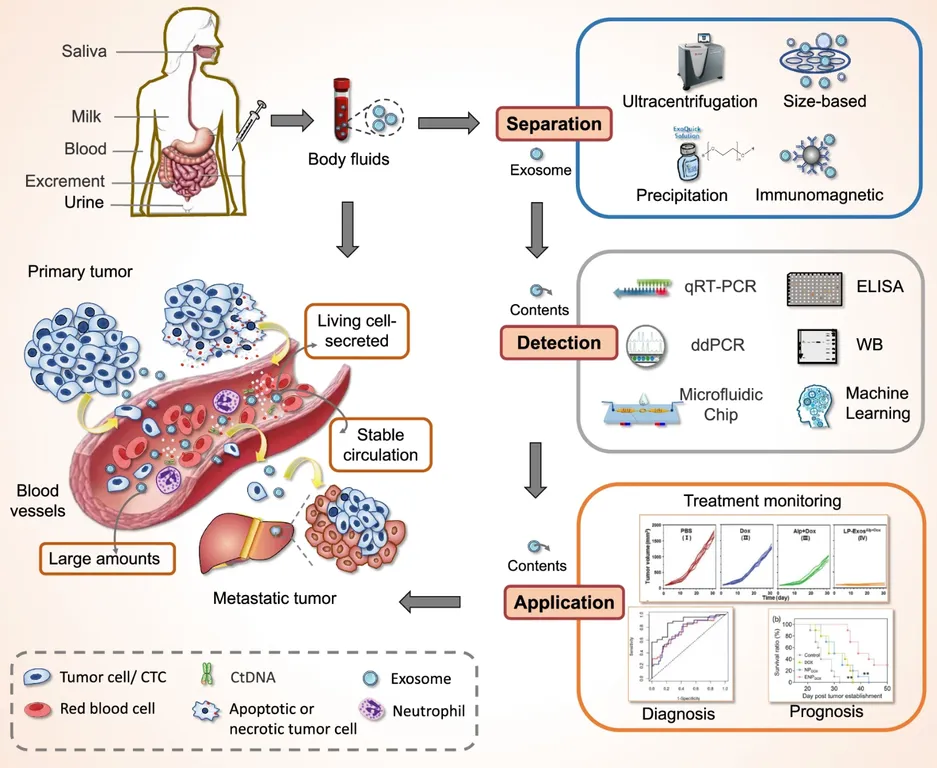
 Figure 2. Protozoa-Derived EV Isolation Project Workflow. (Creative Biostructure)
Figure 2. Protozoa-Derived EV Isolation Project Workflow. (Creative Biostructure)
Sample Requirements
To ensure optimal yield and quality of protozoa-derived extracellular vesicles, Creative Biostructure provides flexible sample processing options and clear submission guidelines. We accept both in vitro protozoan cultures and host-derived biological specimens, with workflows adaptable to aerobic or anaerobic conditions depending on the parasite species and research objectives.
Recommended Submission Guidelines:
- Sample Type: Culture supernatants, tissue homogenates, or biofluids from infected hosts
- Minimum Volume: 10-50 mL of culture supernatant or equivalent volume of homogenized sample
- Storage Conditions:
- 4°C for short-term storage (less than 24 hours)
- -80°C for long-term preservation
- Shipping Conditions:
- Use ice packs for samples stored at 4°C
- Ship on dry ice for frozen materials
We routinely work with a wide range of protozoan species, such as:
| Leishmania spp. | Giardia lamblia | Trypanosoma brucei and T. cruzi | Plasmodium falciparum | Toxoplasma gondii |
| Entamoeba histolytica | Naegleria fowleri | Trichomonas vaginalis | Acanthamoeba spp. | Neospora caninum |
If you have a non-listed species or require support with sample preparation, our technical team will provide personalized guidance to ensure compatibility with our EV isolation workflows.
Quality Control and Deliverables
We deliver research-grade protozoa-derived EVs with complete technical documentation. Standard QC includes:
- Nanoparticle Tracking Analysis (NTA): Assessment of vesicle size distribution and particle concentration
- Transmission Electron Microscopy (TEM): Visualization of vesicle morphology to confirm structural integrity
- Western Blotting: Detection of extracellular vesicle markers such as HSP70 and protozoan-specific surface antigens
- Endotoxin Testing: Evaluation of endotoxin levels to ensure suitability for immunological applications
- Protein and RNA Quantification: Measurement of total protein by BCA assay and RNA quality assessment for downstream omics studies
- Technical Report: A comprehensive document detailing the isolation method, sample input, yield, and all QC results
Case Study
Case: Stage-Specific Isolation of Plasmodium falciparum-Derived Extracellular Vesicles
Background
Researchers investigated extracellular vesicles (EVs) secreted by Plasmodium falciparum-infected red blood cells to explore their protein composition and impact on parasite invasion.
Methods:
EVs were isolated from synchronized early and late-stage cultures of four P. falciparum strains using:
- 21,000 × g centrifugation for microvesicles (MV)
- 110,000 × g ultracentrifugation for exosomes (Exo)
- Filtration and PBS washing steps
Characterization included:
- TEM confirming cup-shaped morphology (~100 nm)
- NTA showing Exo size ~98 nm, MV ~160 nm
- Western blot detecting flotillin-1, stomatin, β-actin
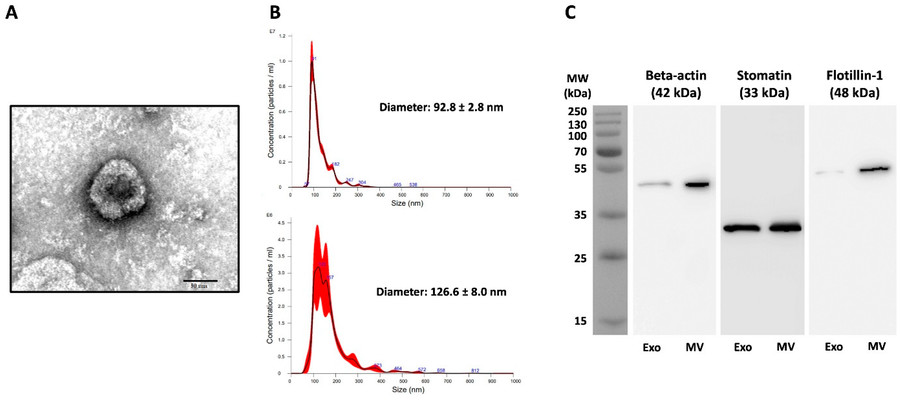 Figure 3. Characterization of Extracellular Vesicles from Plasmodium falciparum Cultures. (A) TEM image of negatively stained Pf-Exo from P. falciparum 3D7. (B) NTA profiles of Pf-Exo (top) and Pf-MV (bottom) showing size distribution. (C) Western blot analysis of β-actin, stomatin, and flotillin-1 in Pf-Exo and Pf-MV isolated from infected red blood cells. (Vimonpatranon S, et al., 2022)
Figure 3. Characterization of Extracellular Vesicles from Plasmodium falciparum Cultures. (A) TEM image of negatively stained Pf-Exo from P. falciparum 3D7. (B) NTA profiles of Pf-Exo (top) and Pf-MV (bottom) showing size distribution. (C) Western blot analysis of β-actin, stomatin, and flotillin-1 in Pf-Exo and Pf-MV isolated from infected red blood cells. (Vimonpatranon S, et al., 2022)
Results:
- Over 150 human proteins and 128 parasite proteins were identified in Pf-EVs
- Key invasion-related proteins such as AMA-1, MSP-1, and EBA-175 were consistently present
- High-dose Pf-MVs slightly reduced parasite growth in vitro, supporting a role in density regulation
Conclusion:
This study demonstrated a reliable EV isolation workflow using stage-specific culture supernatants and differential centrifugation, enabling downstream functional and proteomic analyses of parasite-derived vesicles.
Creative Biostructure is your trusted partner in protozoa-derived EV research. Whether you aim to explore host-pathogen interactions, discover diagnostic biomarkers, or develop vaccine candidates, our tailored EV isolation services will help you achieve reliable and reproducible results. Contact us to discuss your project requirements and receive expert support from our team.
References
- Coakley G, Maizels R M, Buck A H. Exosomes and other extracellular vesicles: the new communicators in parasite infections. Trends in Parasitology. 2015, 31(10): 477-489.
- Khosravi M, Mirsamadi E S, Mirjalali H, et al. Isolation and functions of extracellular vesicles derived from parasites: the promise of a new era in immunotherapy, vaccination, and diagnosis. International Journal of Nanomedicine. 2020: 2957-2969.
- Wang X, Chen J, Zheng J. The state of the art of extracellular vesicle research in protozoan infection. Frontiers in Genetics. 2022, 13: 941561.
- Vimonpatranon S, Roytrakul S, Phaonakrop N, et al. Extracellular vesicles derived from early and late stage Plasmodium falciparum-infected red blood cells contain invasion-associated proteins. Journal of Clinical Medicine. 2022, 11(14): 4250.
Frequently Asked Questions
For any inquiries, our support team is ready to help you get technical support for your research and maximize your experience with Creative Biostructure.